-
PDF
- Split View
-
Views
-
Cite
Cite
Sonia Minuzzo, Valentina Agnusdei, Irene Pusceddu, Marica Pinazza, Lidia Moserle, Massimo Masiero, Elisabetta Rossi, Marika Crescenzi, Timothy Hoey, Maurilio Ponzoni, Alberto Amadori, Stefano Indraccolo, DLL4 regulates NOTCH signaling and growth of T acute lymphoblastic leukemia cells in NOD/SCID mice, Carcinogenesis, Volume 36, Issue 1, January 2015, Pages 115–121, https://doi.org/10.1093/carcin/bgu223
Close - Share Icon Share
Abstract
Activation of the NOTCH pathway occurs commonly in T acute lymphoblastic leukemia (T-ALL) mainly due to mutations in NOTCH1 or alterations in FBW7 and is involved in the regulation of cell proliferation and survival. Since mutations hit different domains of the receptor, they are predicted to heterogeneously perturb ligand-induced NOTCH1 activity. Moreover, T-ALL cells also co-express NOTCH3 receptors which could be triggered by different ligands. In this study, we aimed to investigate the role of DLL4 in the regulation of NOTCH signaling in T-ALL cells in the context of different types of NOTCH1 mutation or wild-type NOTCH receptor, as well as the effects of DLL4 neutralization on T-ALL engraftment in mice.
We found that NOTCH signaling can be stimulated in T-ALL cells in vitro by either human or murine DLL4 with heterogeneous effects, according to NOTCH1/FBW7 mutation status, and that these effects can be blocked by antibodies neutralizing DLL4, NOTCH1 or NOTCH2/3. In vivo, DLL4 is expressed in the spleen and the bone marrow (BM) of NOD/SCID mice bearing T-ALL xenografts as well as the BM of T-ALL patients. Importantly, DLL4 blockade impaired growth of T-ALL cells in NOD/SCID mice and increased leukemia cell apoptosis. These results show that DLL4 is an important component of the tumor microenvironment which contributes to the early steps of T-ALL cell growth.
Introduction
T-acute lymphoblastic leukemia (T-ALL) is a very heterogeneous disease, characterized by several genetic alterations and polymorphic clinical features in children and adults (1). Notably, about 60% of T-ALL shows increased NOTCH1 activity (2), due to activating NOTCH1 mutations or alterations in FBW7, an ubiquitin ligase responsible for NOTCH1 intracellular domain (ICD) priming for degradation (3,4). NOTCH1 mutations typically occur in two domains of the receptor. Those affecting the heterodimerization domain (HD, a juxta-membrane domain involved in the first step of NOTCH1 cleavage) result in increased activity of the γ-secretase complex by facilitating its access to the NOTCH molecule and hence, increasing the production of the ICD moiety (5). Conversely, mutations affecting the intracellular C-terminal PEST domain (containing sequences responsible for ICD ubiquitination and degradation) increase the half-life of the NOTCH signaling unit and eventually increase the signaling activity. Notably, HD and PEST mutations are not mutually exclusive, and occur simultaneously in about 10–20% of T-ALL patients (6).
Constitutive activation of this signaling pathway confers to the cell a strong growth advantage due to secondary effects on key regulators of cell proliferation, such as c-myc and p27 (7–9). In addition, NOTCH activation can perturb the balance between apoptosis and survival towards the latter through different mechanisms, including increased NFkB signaling (10), increased availability of the X-linked inhibitor of apoptosis protein (XIAP)—due to interference with its ubiquitination and degradation (11)—and activation of PKB/AKT/mTOR pathway-mediated p53 inhibition (12). Moreover, we recently demonstrated an additional mechanism of apoptosis control downstream of NOTCH3, involving the MKP-1 phosphatase and inhibition of p38 and JNK activation (13).
Deregulation of NOTCH3 signaling has also been proposed to be important in T-ALL in view of the oncogenic potential of the NOTCH3 ICD in transgenic mouse models (14). Since NOTCH3 mutations have not been reported in T-ALL (15), it is reasonable to speculate that signaling downstream of this particular NOTCH receptor in T-ALL cells could be ligand-dependent. In this regard, activation of NOTCH3 by its ligand DLL4—expressed on angiogenic vessels—has recently been shown to co-ordinate escape of tumor cells from quiescence, leading to progressive tumor growth in a xenograft model of T-ALL (16) and in colorectal cancer models (17).
DLL4 is constitutively expressed in some lymphoid and hematopoietic organs (including thymus, spleen and bone marrow [18–20]), where it is influential in the regulation of NOTCH signaling during hematopoiesis and T-cell development (21). Moreover, DLL4 is expressed in the microenvironment of solid tumors (22,23) and in T lymphoblastic lymphoma (16), suggesting that this ligand could retain its regulatory role on NOTCH signaling even in pathologic conditions. This is also suggested by the observation that over-expression of DLL4 induces a T-cell lymphoproliferative disease restricted to bone marrow (BM) and lymphoid organs, indicating that excessive activation of NOTCH signaling by DLL4 alters the proliferative and/or apoptotic properties of developing T cells (20).
In this study, we investigated the role of DLL4 in the regulation of NOTCH signaling in T-ALL cells in the context of different types of NOTCH1 mutation or wild-type NOTCH receptor, as well as the effects of DLL4 neutralization on T-ALL engraftment in mice.
Materials and methods
T-ALL xenografts establishment and in vitro cell culture.
Primary T-ALL cells (PDTALL) were obtained from BM of newly diagnosed ALL pediatric patients, according to the guidelines of the local ethics committees. Xenografts establishment and their genetic characterization are reported elsewhere (24). In vitro studies were performed with T-ALL cultures established from the spleen of the xenografts. Purity of the cultures (in terms of percentage of human CD5+ cells) was checked by flow cytometry and was always greater than85%. PDTALL cells were grown in vitro in MEM alpha medium (Life Technologies, Paisley, UK), supplemented with 10% fetal calf serum (FCS), 10% human heat inactivated AB+ serum, 1% penicillin/streptomycin, 1% Glutamax (all from Life Technologies), human IL7 (10ng/ml), human SCF (50ng/ml), human FLT3-ligand (20ng/ml) (all from Peprotech, London, UK) and insulin (20nM) (Sigma–Aldrich, St Louis, MO). In a set of experiments, we coated 12 well-plates with soluble recombinant murine or human DLL4 (1.6 µg/well; both from R&D Systems, Minneapolis, MN) in PBS–BSA 0.1%; one day later, T-ALL cells were seeded (2.5 × 106/well) and cultured for 48h before cell harvesting and western blot or RNA extraction and transcriptional analysis. Primary T-ALL cells were treated with anti-murine DLL4 (OMP-21R30 [25]), anti-hNOTCH1 (OMP-52M51 [24]) or anti-hNOTCH2/3 (OMP-59R5 [26]) monoclonal antibodies (mAbs) developed by OncoMed Pharmaceuticals (Redwood City, CA) in collaboration with MorphoSys AG (Martinsried, Germany [27]). The negative control was OMP-1B711, a murine mAb directed against dinitrophenol (also known as anti-hapten) used in previous studies (25). All mAbs were used at final concentration of 10 μg/ml and were added to the cultures for the entire duration of the assay (48h).
Tumorigenicity assay.
NOD/SCID mice were purchased from Charles River (Wilmington, MA). Procedures involving animals and their care conformed to institutional guidelines that comply with national and international laws and policies (EEC Council Directive 86/609, OJ L 358, 12 December, 1987) and were authorized by the ethical committee of the University of Padova. T-ALL cells engraftment was monitored by periodic blood drawings and flow cytometric analysis of CD5 marker. To test the effect of DLL4 blockade on leukemia growth, NOD/SCID mice were intraperitoneally (i.p.) injected with the anti-murine DLL4 mAb OMP-21R30 or control mAb OMP-1B711 one day before T-ALL cells. mAbs were dosed (both 10mg/Kg) as previously reported (28) and administered every 2–3 days. In all experiments, mice were inspected twice weekly to detect early signs and symptoms of leukemia and blood was weekly drawn to measure T-ALL cell engraftment.
Immunohistochemistry.
For immunohistochemical analysis, 5 μm thick formalin-fixed and paraffin-embedded spleen sections were incubated with rabbit anti-DLL4 polyclonal antibody from Abcam (ab7280, Abcam, Cambridge, UK) according to the manufacturer’s instructions. This antibody was previously validated in immunohistochemistry (IHC) studies by others (29). The specificity of the staining procedure was confirmed by replacing the primary Ab with PBS.
Immunofluorescence.
For immunofluorescence analysis, frozen spleen sections were incubated with rat anti-MECA-32 (mouse pan-endothelial cell antigen, BD Pharmingen, San Jose, CA) primary antibody, washed and incubated with anti-rat Alexa Fluor 488 secondary antibody, whereas nuclei were stained with TO-PRO-3 iodide (both from Life Technologies). Confocal laser scanning microscopy was carried out with a Zeiss LSM 510 microscope using Argon (488nm) and helium-neon (543–633nm) laser sources. The number of fields analysed varied between 4 and 8 per section, depending on sample size. Due to the physiological abundance of blood vessels in the spleen, which would render standard measurement of microvessel density (MVD) inaccurate, vascularity was analysed by morphometric quantification of MECA-32+ areas.
Western blot analysis.
Cell lysates were run on 4–12% polyacrylamide gels; separated proteins were then blotted for 2h at 400 mA onto a nitrocellulose membrane. Immunoprobing was performed using: rabbit mAb against NOTCH1 ICD (Val1744–D3B8, Cell Signaling Technologies, Beverly, MA), rabbit polyclonal Ab against NOTCH3 (Abcam, Cambridge, UK) and rabbit polyclonal anti-β-actin (Sigma–Aldrich), followed by hybridization with a 1:5000 diluted horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse Ab (Perkin Elmer, Waltham, MA). Antigens were detected by luminescent visualization using the Western Lightning Plus ECL (Perkin Elmer). Signal intensity was measured using a Biorad XRS chemiluminescence detection system.
Reverse transcription and quantitative PCR.
Total RNA was isolated using TRIzol® Reagent according to manufacturer’s instructions. cDNA was synthesized from 1–1.5 μg of total RNA using High Capacity RNA-to-cDNA kit (Life Technologies). For quantitative PCR (qPCR) analysis, the SYBR Green dye was used. Primers used for qPCR analysis are reported in Supplementary Table I, available at Carcinogenesis online. For analysis of the NOTCH pathway activation, 21 NOTCH target genes were evaluated in duplicates by Custom TaqMan® Array Cards using TaqMan® Universal PCR Master Mix as reported elsewhere (24). All qPCR reactions were performed using an ABI Prism 7900 Sequence Detection System; relative quantification was done using the ΔΔCt method normalizing to huβ2-microglobulin or muβ-actin mRNA. Relative expression levels of Notch target genes were normalized by setting at 1 the BSA sample. All reagents were obtained from Life Technologies.
Flow cytometry analysis.
Fluorescein isothiocyanate (FITC)-labeled mAb against CD5 (Coulter, Fullerton, CA) was used for the detection of T-ALL cells in mouse samples. The Annexin-V-FLUOS Staining Kit (Roche Diagnostics, Penzberg, Germany) was used to evaluate apoptosis. Samples were analysed on a Beckman Coulter EPICS-XL Flow Cytometer (Coulter) or BD LSRII Flow Cytometer (BD Biosciences).
Statistical analysis.
Results were expressed as mean value ± SD. Statistical analysis of data was performed using Student’s t test. Differences were considered statistically significant when P < 0.05.
Results
Generation and genetic characterization of T-ALL xenografts.
We first analysed the effects of DLL4 on NOTCH signaling in T-ALL cells representative of the various types of mutations found in patients. Accordingly, we established n = 19 leukemia xenografts by intravenous (i.v.) injection of T-ALL cells (1 × 107 cells/mouse) freshly obtained from human bone marrow samples into NOD/SCID mice. Molecular analysis disclosed that all xenografts retained the same T-cell receptor rearrangement found in the primary leukemic samples and had heterogeneous NOTCH1 and FBW7 genetic status. These results as well as additional data about patient’s age, T-ALL phenotype, minimal residual disease (MRD) class risk and prednisone sensitivity have been recently published (24). Nine of these T-ALL cases, bearing various types of NOTCH1/FBW7 mutations (Supplementary Table II, available at Carcinogenesis online), were used for this study.
DLL4 and other NOTCH ligands are expressed in the tumor microenvironment.
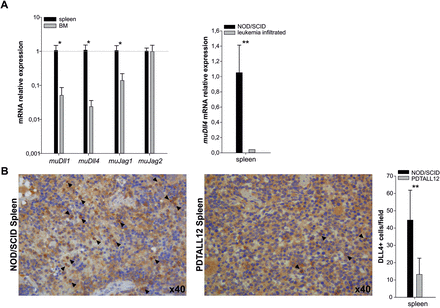
Expression of DLL4 in lymphoid and hematopoietic organs has been described by previous studies (18–20). Accordingly, the Dll4 transcript was detected both in the spleen and the BM of NOD/SCID mice. Based on qPCR data, however, Dll4 expression was more abundant in the spleen compared with the BM (Figure 1A). Intriguingly, Dll4 transcript levels were much reduced in the spleen of PDTALL xenografts, compared to healthy mice (Figure 1A). With regard to other NOTCH ligands, Jagged-1 and Dll1 expression was detected at higher levels in spleen compared with BM samples, whereas Jagged-2 was expressed at comparable levels in these two tissues (Figure 1A). DLL4 protein was expressed in the spleen (Figure 1B) by stromal cells as well as hematopoietic cells. In contrast, substantial reduction of DLL4+ cells was observed in the spleen of mice xenografted with human T-ALL cells—probably due to the massive replacement of the normal tissue by leukemic blasts—albeit strong positivity was observed in some residual murine cells (Figure 1B).
Expression of NOTCH ligands in the tumor microenvironment. (A). Measurement of murine (mu) Dll1, Dll4, Jag1 and Jag2 expression by qPCR analysis in BM and spleen of NOD/SCID mice. Murine β-actin was used as housekeeping gene. Left panel: Expression levels of each ligand in the BM were normalized to their expression in the spleen (mean ± SD of n = 4 samples). Right panel: Expression levels of muDll4 transcript in the spleen of PDTALL xenografts compared to healthy spleen (mean ± SD of n = 4 samples). (B) IHC analysis of DLL4 expression in spleen of a NOD/SCID healthy mouse (left) and of a mouse xenografted with PDTALL12 leukemia cells (middle) using a rabbit anti-DLL4 polyclonal antibody from Abcam (ab7280). Two representative high magnification (×40) images are reported. Positive cells are indicated by arrowheads. The histogram represents mean ± SD values of DLL4+ cells in the spleen of mice; quantitative analysis was performed at magnification ×20 by scoring n = 10 representative fields, n = 3 samples per group. Statistically significant differences are indicated (*P < 0.05, **P < 0.001).
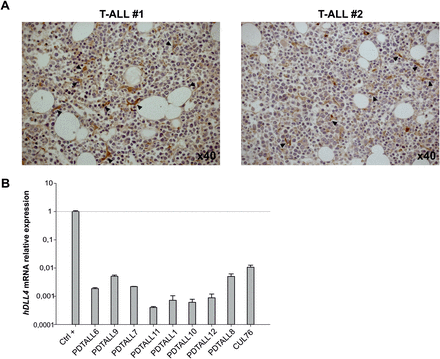
Importantly, DLL4 protein was also found expressed in the BM of T-ALL patients, mainly by cells with endothelial or dendritic cell-like morphology (Figure 2A); thus, reinforcing the clinical relevance of our observations. To investigate whether also leukemia cells might express DLL4, we analysed by qPCR the expression of its transcript in T-ALL samples. Results clearly show that human DLL4 is barely expressed by any of the xenografts tested (Figure 2B), therefore confirming its expression only by host cells of the tumor microenvironment.
DLL4 expression in human T-ALL samples. (A) Evaluation of DLL4 expression by IHC in human BM samples from two T-ALL patients. Positive cells are indicated by arrowheads. (B) Quantitative real-time-PCR analysis of human DLL4 transcript in T-ALL xenografts. Human umbilical vein endothelial cells (HUVEC) were used as positive controls (Ctrl+) and human β2-microglobulin was used as housekeeping gene. Expression levels were normalized to Ctrl+.
DLL4 can stimulate NOTCH signaling in vitro.
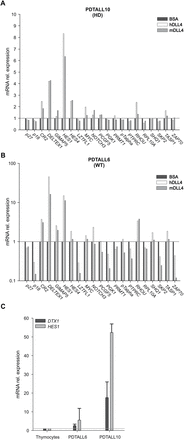
We next investigated whether recombinant murine or human DLL4 could stimulate NOTCH signaling in primary T-ALL cells from xenografts. In the initial experiment, relative induction of NOTCH target transcripts was substantially higher in T-ALL cells with wild-type NOTCH1 sequences (PDTALL6) than in a T-ALL sample (PDTALL10) bearing a NOTCH1 mutation in the HD domain and a FBW7 mutation (Figure 3A and B). This result is probably due to the fact that certain NOTCH1 mutations raise endogenous NOTCH signaling, therefore lowering the stimulating potential of exogenous NOTCH ligands. In keeping with this hypothesis, basal endogenous levels of DELTEX1 and HES1 transcripts were much higher in PDTALL10 compared with PDTALL6 cells (Figure 3C). Among the 21 NOTCH target genes evaluated, HES1 and DELTEX1 were strongly up-regulated by DLL4 in both primary cases and were therefore selected to analyse additional T-ALL samples.
NOTCH signaling is stimulated by DLL4 in vitro. (A–B)TaqManArrays were utilized to investigate effects on expression levels of 21 NOTCH-target genes following 48h cultivation on human or murine DLL4-coated wells. Gene expression profiles of a NOTCH1 mutated (PDTALL10) and of a NOTCH1 wild-type (PDTALL6) xenograft are shown. (C) Expression of NOTCH target genes DELTEX1 and HES1 correlates with the genomic mutational status in NOTCH1/FBW7 genes (mean ± SD of 3 independent biological replicates). Relative expression levels of NOTCH target genes were determined by quantitative real-time PCR and normalized by setting at 1 the value detected in normal thymocytes (n = 4 samples).
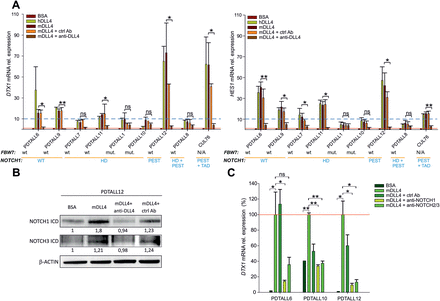
Altogether, experiments with ex vivo cultures from various T-ALL xenografts (n = 9) confirmed that NOTCH signaling could be stimulated by DLL4 in vitro. In general, similar results were obtained following co-culturing T-ALL cells with murine or human DLL4-coated wells, although in individual cultures either the human or the mouse ligand exerted stronger stimulatory effects (Figure 4A). Strong responses to DLL4 stimulation—defined by an arbitrary relative increase of both DELTEX1 and HES1 levels greater than 10—were measured in T-ALL samples bearing parental NOTCH1 sequences (PDTALL6 and 9) as well as samples bearing mutated NOTCH1 sequences (PDTALL12 and CUL76). With regard to NOTCH1 mutated samples, there was some correlation between type of NOTCH1 mutation and intensity of stimulation by DLL4. Indeed, strong induction of DELTEX1 and HES1 transcripts was measured in CUL76 and PDTALL12 cells, which carry PEST+TAD and PEST mutations, respectively. In contrast, T-ALL samples bearing mutations in the HD domain (PDTALL1, 7, 10, 11) or mutations in the HD and PEST domains (PDTALL8) were relatively less responsive to DLL4 stimulation. Fbw7 mutations were found in two xenografts (PDTALL1 and 10) bearing HD mutations and associated with relatively low responsiveness to DLL4. Finally, murine DLL4-blocking antibodies strongly reduced (generally by greater than 80%) upregulation of NOTCH target transcripts, thus showing the specificity of the phenomenon observed (Figure 4A).
NOTCH signaling is modulated by neutralizing antibodies in vitro. (A) Expression levels of DELTEX1 (left) and HES1 (right) transcripts in xenografts bearing different NOTCH1/Fbw7 mutations. Cells (2.5×106/well) were cultivated for 48h in DLL4-coated wells. In some wells, anti-DLL4 or a control antibody (ctrl Ab) were added to the medium at final concentration of 10 μg/ml. Mean values ± SD of three replicates are reported. Red line: DELTEX1 and HES1 expression levels in control cultures (BSA). Blue dotted line: arbitrary cut-off used to stratify transcriptional responses. (B). Western blot analysis of NOTCH1 and NOTCH3 ICD levels in PDTALL12 cells cultured in mDLL4-coated wells with or without anti-DLL4 neutralizing antibodies. Numbers below the bands indicate the ratio between the indicated protein and β-actin bands normalized by setting at 1 the value assigned to the BSA sample. (C) Evaluation of DELTEX1 mRNA in PDTALL6, PDTALL10 and PDTALL12 T-ALL cells cultivated on mDLL4-coated wells for 48h and treated with either anti-NOTCH1, anti-NOTCH2/3 or control antibodies. Red line: DELTEX1 expression levels in murine DLL4-stimulated cultures. Statistically significant differences are indicated (*P < 0.05, **P < 0.001).
Western blot analysis confirmed increased levels of NOTCH1 ICD, as well as a minor increase in NOTCH3 ICD following DLL4 stimulation and these changes were prevented by DLL4 neutralization (Figure 4B). Finally, treatment with either anti-NOTCH1 or anti-NOTCH2/3 mAbs strongly attenuated (range 64.1–90.6%) effects of DLL4 stimulation on DELTEX1 expression, indicating that these NOTCH receptors are involved in transduction of DLL4 signals (Figure 4C).
Anti-murine DLL4 impairs growth of T-ALL cells in vivo.
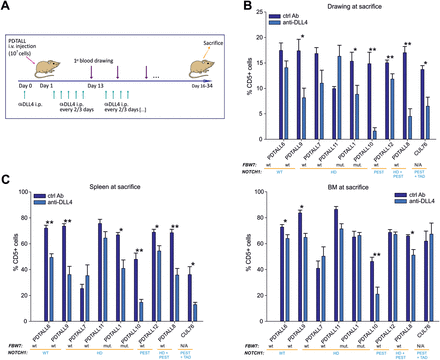
The finding that DLL4 stimulation triggered NOTCH signaling in T-ALL cells prompted investigation of the relevance of this interaction in vivo. Since DLL4 is poorly expressed in organs already heavily infiltrated by T-ALL blasts (Figure 1B), we sought to investigate this interaction in a preventive setting. To this end, NOD/SCID mice were injected on day 1 with anti-murine DLL4 mAb, followed by i.v. injection of 1 × 107 T-ALL cells one day later. Anti-DLL4 mAb administration was repeated every 2–3 days up to sacrifice of control mice, 16–34 days from the injection of T-ALL cells (Figure 5A). This experiment was performed with all the primary T-ALL xenografts bearing either parental or mutated NOTCH1 alleles.
DLL4 neutralization delays engraftment of T-ALL cells in vivo. (A). Outline of treatment with anti-DLL4 (OMP-21R30) or control Ab (OMP-1B711). NOD/SCID mice (n = 6 mice/group) were i.p. treated with anti-DLL4 or ctrl Ab one day before T-ALL cells injection (107 cells/mouse) and subsequently every 2–3 days until sacrifice. (B). Measurements of circulating CD5+ blasts by flow cytometry at sacrifice. (C). Quantification of CD5+ leukemia cells in spleen (left panel) and BM (right panel) at sacrifice. Statistically significant differences are indicated (*P < 0.05, **P < 0.001).
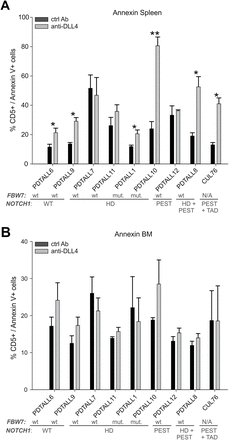
Engraftment of T-ALL was monitored by flow cytometric analysis of human T-ALL cells in the peripheral blood of the mice at consecutive time points or in the spleen and the BM at sacrifice. Results indicate that anti-DLL4 mAb significantly reduced CD5+ cells in the peripheral blood of the mice in all cases analysed, except PDTALL6, PDTALL7 and PDTALL11 (Figure 5B). Moreover, DLL4 neutralization reduced the percentage of human blasts in the spleen of the mice, whereas less marked effects were observed in the BM (Figure 5C). Overall, reduction of blasts in the blood, spleen and BM of mice treated with anti-DLL4 mAb compared with controls was 21–89%, 21–69% and 2.3–54.5%, respectively. Best responders to DLL4 blockade—defined by significant differences in the percentage of T-ALL blasts in all districts analysed (blood, BM and spleen)—included PDTALL8, 9 and 10. Intermediate responders—showing reduction of blasts in 2 out of 3 districts analysed—included PDTALL1, 6, 12 and CUL76. Finally, PDTALL7 and PDTALL11, two xenografts bearing an HD mutation in NOTCH1, were the only non-responders (Figure 5). Importantly, T-ALL cells from the spleen of anti-DLL4 treated mice showed increased level of apoptosis when compared with control mice (except PDTALL7, 11 and 12 xenografts), but such a difference was not found for BM-infiltrating leukemia cells from the same mice (Figure 6). No clear-cut correlation between NOTCH1/Fbw7 genetic status and response to DLL4 blockade in vivo was observed, although both anti-DLL4 resistant xenografts were characterized by NOTCH1 mutations in the HD domain.
Analysis of apoptosis in leukemic cells after anti-DLL4 treatment. At sacrifice, apoptosis was evaluated in spleen (A) and BM (B) by annexin V and CD5 labelling and flow cytometric analysis. Statistically significant differences are indicated (*P < 0.05, **P < 0.001).
Discussion
This study investigated to which extent NOTCH mutations, which are found in the majority of T-ALL samples, make leukemia cells refractory to stimulation by DLL4, a NOTCH ligand with an established role in the regulation of NOTCH signaling in T cell physiology (21).
In vitro results showed that several NOTCH target genes are increased by DLL4 stimulation in T-ALL cells, including CR2, DELTEX1, GIMAP5, HES1, HES4 and RHOU. We performed extensive studies by measurement of DELTEX1 and HES1 transcript levels, as their variations following DLL4 stimulation were particularly strong, and also based on our previous study showing that these genes are reliable markers of NOTCH signaling in T-ALL cells (24). According to these results, although some degree of stimulation was observed in all cases, best in vitro responders to DLL4 stimulation—defined by an arbitrary relative increase of DELTEX1 and HES1 levels graeter than 10—were T-ALL cells with either wild-type NOTCH1 sequences (such as PDTALL6 and PDTALL9) or with mutations in the PEST domain (such as PDTALL12 and CUL76). T-ALL cells with mutations in the HD domain with or without concomitant mutations in FBW7 could also be stimulated in vitro by DLL4 but to a lower degree, suggesting that in these cells ligand-independent NOTCH signaling prevails. These results confirm conclusions of a previous study that analysed by reporter gene assays effects of different HD mutations on ligand-dependent NOTCH signaling (5). Moreover, they are also in-line with the established observation that coculture of primary human T-ALL cells, displaying either mutated or naive Notch receptor, with a mouse stromal cell line expressing the DLL1 ligand permits maintenance of T-ALL cells and long-term growth of blast cells (30). Stimulation of NOTCH signaling in HD-mutated T-ALL cells could depend both on NOTCH1 and NOTCH3, which is expressed by these cells and contributes in part to transduction of DLL4 signals, based on results of receptor neutralization assays (Figure 4C).
In vivo studies showed that DLL4 blockade delayed leukemia growth, although it did not impact on T-ALL cell engraftment. The finding that the mouse BM and spleen express also other NOTCH ligands, such as Dll1, Jagged-1 and -2 (Figure 1A), may account for the partial effects of DLL4 blockade on leukemia growth. In this regard, it has been recently published that Jagged-1 expressed in the tumor vascular niche can support NOTCH signaling in neighboring lymphoma cells (31).
Notably, in our study anti-leukemia effects of DLL4 blockade were largely independent from the genetic status of NOTCH1 in T-ALL cells. In fact, best in vivo responders were PDTALL8 and 10, two T-ALL xenografts bearing HD+PEST and HD mutations, respectively, along with PDTALL9, a xenograft with wild-type NOTCH1 sequences. This result suggests that DLL4 blockade could limit leukemia outgrowth in vivo by mechanisms in part independent from levels of endogenous NOTCH signaling in T-ALL cells and supports the hypothesis that the stromal environment is important to modulate NOTCH signaling in leukemia cells (32). The link between the Notch pathway, which is pivotal for T-ALL onset and development, and NOTCH ligand positive stromal cells, which are necessary for preventing blast cell apoptosis, could probably rely on the growth factors that stromal cells produce and release in presence of neoplastic lymphoid cells. For instance, T-ALL cells in contact with BM stromal cells are rescued from apoptosis through an IL-7–dependent mechanism (33). On the other hand, it should be noted that PDTALL7 and 11, the two T-ALL xenografts which did not respond to anti-DLL4 therapy, shared the same L1679P mutation in the C-terminal region of the HD domain of NOTCH1 (Supplementary Table II, available at Carcinogenesis online). Previous studies showed that most leukemia-associated NOTCH1 HD domain mutations (including L1679P) activate NOTCH1 independently from stimulation by canonical ligands, but with heterogeneous strength depending on the particular HD domain mutation (5). Therefore, it is tempting to speculate that certain HD mutations might confer resistance to DLL4 blockade in vivo.
Among other targets of anti-DLL4 treatment, we were aware of the effects of DLL4 blockade on the tumor vasculature in solid tumors (34,35). However, in our T-ALL models, we did not detect obvious variations in the number of blood vessels or their size in the spleen of mice bearing T-ALL cells treated with anti-DLL4 (Supplementary Figure 1, available at Carcinogenesis online), apparently not supporting anti-angiogenic effects of DLL4 blockade in this experimental system. This result may be due to intrinsic differences in the vasculature of the spleen compared with that of solid tumors. In any case, given the importance of DLL4-NOTCH signaling in the endothelium, it is conceivable that systemic inhibition of DLL4, as would occur with anti-DLL4 therapy, might affect angiogenesis, especially in the BM. In this regard, it has been shown that endothelial NOTCH activity promotes angiogenesis and osteogenesis in murine bone (36). Therefore, anti-DLL4 therapy could exert anti-angiogenic effects especially in this district and modulate both physiological processes, such as osteogenesis, and leukemia engraftment. Prospectively, this should be considered in the case of clinical application of anti-DLL4 antibodies.
Moreover, systemic anti-DLL4 treatment was recently reported to perturb the BM vascular niche, leading to changes of angiocrine factors expression (including increased IGFbp2, IGFbp3, Angpt2, DHH, VEGF-A and decreased FGF1 and CSF2 expression) and also to perturbation of hematopoiesis in mice (37). Therefore, in view of the fact that vascular niche-derived paracrine factors are known to enforce aggressiveness of lymphoid malignant cells (31), it is possible that the anti-leukemia effect of anti-DLL4 therapy might in part depend on modulation of angiocrine factors in the murine microenvironment. Future studies are needed to substantiate this possibility.
In conclusion, our study showed that direct effects of DLL4 on NOTCH signaling in T-ALL cells can consistently be found in vitro, especially in cells bearing wild-type NOTCH1 receptors or mutations in the PEST domain. Moreover, DLL4 blockade delays growth of T-ALL cells and increases their apoptosis, an effect possibly mediated by a combination of direct effects on NOTCH signaling in T-ALL cells and indirect effects on the tumor microenvironment. Prospectively, these results could have implications also for other types of leukemia, such as B-CLL, where NOTCH1 mutations in the PEST domain have recently been described (38) and where the role of the microenvironment is consolidated (39).
Supplementary material
Supplementary Figure 1 and Tables I–II can be found at http://carcin.oxfordjournals.org/
Funding
Associazione Italiana per la Ricerca sul Cancro (AIRC Project n. IG14295) and Fondazione Italiana per la Ricerca sul Cancro (FIRC); Ministry of University and Research, 60% and PRIN; Foundation CARIPARO Projects of Excellence Program 2008.
Abbreviations:
- BM
bone marrow
- ICD
intracellular domain
- IHC
immunohistochemistry
- mAbs
monoclonal antibodies
- PDTALL
primary T-ALL cells
- T-ALL
T-acute lymphoblastic leukemia
- qPCR
quantitative PCR.
Acknowledgements
We are grateful to Adolfo Ferrando (Columbia University, New York, NY) for providing the CUL76 xenograft; Giovanni Esposito (Istituto Oncologico Veneto) for evaluation of IHC studies; Nazarena Nannini (Department of Cardiac, Thoracic and Vascular Sciences, University of Padova) for quantification of MECA-32+ areas.
Conflict of Interest Statement: T.H. is employee of OncoMed Pharmaceuticals. S.I. received research funding from Oncomed Pharmaceuticals. The other authors declare no conflict of interest.
References