-
PDF
- Split View
-
Views
-
Cite
Cite
Ajita V. Singh, Dong Xiao, Karen L. Lew, Rajiv Dhir, Shivendra V. Singh, Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo, Carcinogenesis, Volume 25, Issue 1, January 2004, Pages 83–90, https://doi.org/10.1093/carcin/bgg178
Close - Share Icon Share
Abstract
Sulforaphane (SFN), a constituent of cruciferous vegetables, is highly effective in affording protection against chemically induced cancers in animal models. Here, we report that SFN inhibited proliferation of cultured PC-3 human prostate cancer cells by inducing apoptosis that was characterized by appearance of cells with sub-G 0 /G 1 DNA content, formation of cytoplasmic histone associated DNA fragments and cleavage of poly(ADP-ribose)polymerase (PARP). SFN-induced apoptosis was associated with up-regulation of Bax, down-regulation of Bcl-2 and activation of caspases-3, -9 and -8. SFN-induced apoptosis, and cleavage of procaspase-3 and PARP were blocked upon pre-treatment of cells with pan caspase inhibitor z-VADfmk, and specific inhibitors of caspase-9 (z-LEHDfmk) and caspase-8 (z-IETDfmk) suggesting involvement of both caspase-9 and caspase-8 pathways in SFN-induced cell death. Oral administration of SFN (5.6 µmol, 3 times/week) significantly inhibited growth of PC-3 xenografts in nude mice. For instance, 10 days after starting therapy, the average tumor volumes in control and SFN-treated mice were 170 ± 13 and 80 ± 14 mm 3 , respectively, reflecting a >50% reduction in tumor volume due to SFN administration. To the best of our knowledge, the present study is the first published report to document in vivo anticancer activity of SFN in a tumor xenograft model.
Introduction
Epidemiological studies have suggested that increased dietary consumption of cruciferous vegetables may be protective against the risk for certain types of malignancies, including prostate cancer ( 1 – 5 ). Antineoplastic effects of cruciferous vegetables are credited to isothiocyanates (ITCs) that are generated upon cutting or chewing of these vegetables ( 6 – 9 ). ITCs are highly effective in affording protection against cancer in experimental animals induced by a variety of chemical carcinogens, including tobacco-smoke-derived carcinogens ( 8 – 14 ).
Sulforaphane [SFN; 1-isothiocyanato-4-(methylsulfinyl)-butane; CH 3 -SO-(CH 2 ) 4 -N=C=S)] is a naturally occurring member of the ITC family that has received particular attention not only because it is present in rather large quantities in certain varieties of broccoli but also due to its anticancer effects ( 15 – 17 ). Orally administered SFN significantly inhibited 9,10-dimethyl-1,2-benzanthracene induced mammary tumorigenesis in rats ( 16 ). SFN was shown to offer impressive and sustained protection against oxidative damage in human retinal pigment epithelial cells, keratinocytes and L1210 mouse leukemia cells ( 18 ). An anti-oxidative effect for SFN was also observed in aortic smooth muscle cells from spontaneously hypersensitive rats ( 19 ). In addition, SFN exhibited bactericidal activity against clinical isolates and antibiotic resistant strains of Helicobacter pylori , and afforded significant protection against benzo[ a ]pyrene-induced forestomach cancer in mice ( 20 ). Mechanism for cancer preventive effect of SFN involves modulation of carcinogen metabolism due to inhibition of cytochrome P450-dependent monooxygenases and/or induction of Phase II detoxification enzymes including glutathione transferases and quinone reductase ( 21 – 26 ).
Evidence is mounting to indicate that SFN can inhibit growth of human cancer cells in culture by inducing apoptosis and causing cell cycle arrest ( 27 – 30 ). Growth inhibition, apoptosis induction and/or cell cycle arrest has been observed in SFN-treated HT29, LS-174 and Caco-2 human colon cancer cells ( 27 , 28 ), LNCaP human prostate cancer cells ( 29 ), and leukemia cells ( 30 ). However, the mechanism by which SFN induces apoptosis or cell cycle arrest is not fully defined. Moreover, despite compelling experimental evidence for antiproliferative activity of SFN against cultured cancer cells ( 27 – 30 ), it is not known if SFN can inhibit growth of cancer cells in vivo . Here, we report that SFN suppresses proliferation of PC-3 human prostate cancer cells in culture by inducing caspase-9 and caspase-8-mediated apoptosis. We also demonstrate that oral administration of SFN significantly retards growth of PC-3 xenografts in nude mice at a concentration that may be generated through dietary intake of broccoli and other cruciferous vegetables. In conclusion, the results of the present study strongly argue for a systematic pre-clinical and clinical evaluation of SFN for its activity against human prostate cancer.
Materials and methods
Reagents
d , l -SFN (>99% pure) was purchased from LKT Laboratories (St Paul, MN). PC-3 cell line was a generous gift from Dr Candace S.Johnson (currently at the Roswell Park Cancer Institute, Buffalo, NY). F-12K Nutrient Mixture, penicillin/streptomycin antibiotic mixture, and fetal bovine serum were from Gibco (Grand Island, NY), propidium iodide was from Sigma (St Louis, MO), RNaseA was from Promega (Madison, WI), and the reagents for electrophoresis were from Bio-Rad (Hercules, CA). Antibodies against Bcl-2, Bcl-X L , Bax, Bid and procaspase-8 were from Santa Cruz Biotechnology (Santa Cruz, CA), antibodies specific for cleaved caspase-3 and poly(ADP-ribose)polymerase (PARP) were from Cell Signaling Technology (Beverly, MA), and antibody against cleaved caspase-9 was from BD Pharmingen (San Diego, CA). The caspase inhibitors z-VADfmk (general caspase inhibitor), z-IETDfmk (caspase-8) and z-LEHDfmk (caspase-9) were from Enzyme Systems (Dublin, CA).
Cell culture and cell proliferation assays
Monolayer cultures of PC-3 cells were maintained at 37°C in F-12K Nutrient Mixture supplemented with 7% (v/v) fetal bovine serum and antibiotics in a humidified atmosphere of 5% CO 2 and 95% air. Effect of SFN on proliferation of PC-3 cells was determined by sulforhodamine B and trypan blue dye exclusion assays as described by us previously ( 31 , 32 ).
Determination of apoptosis
Apoptosis induction in SFN-treated PC-3 cells was assessed by (i) quantification of cytoplasmic histone associated DNA fragments, (ii) flow cytometric analysis of cells with sub-G 0 /G 1 DNA content following staining with propidium iodide and (iii) western blot analysis for PARP cleavage. The Cell Death Detection ELISA method quantifies apoptotic cell death in cellular systems by measuring cytoplasmic histone associated DNA fragments. Cytoplasmic histone associated DNA fragments in control (DMSO treated) and SFN-treated cells (20 or 40 µM SFN for 24 h) were quantified using a commercially available ELISA kit from Roche Diagnostics GmbH (Mannheim, Germany). For analysis of cells with sub-G 0 /G 1 DNA content, cells (5 × 10 5 cells) were seeded into T75 flasks, and allowed to attach overnight. The medium was replaced with fresh complete medium containing 20 µM SFN. An equal volume of DMSO was added to controls. Following incubation for 24, 48 or 72 h at 37°C, floating and attached cells were collected, washed with phosphate-buffered saline (PBS) and fixed with 70% ethanol. Fixed cells were then treated with 80 µg/ml RNase A and 50 µg/ml propidium iodide for 30 min, and analyzed using a Coulter Epics XL Flow Cytometer ( 31 ).
Western blot analysis
After treatment with DMSO (control) or desired concentration of SFN for specified time interval, floating and attached cells were collected and lysed as described by us previously ( 31 , 32 ). Cell lysate was cleared by centrifugation at 14 000 r.p.m. for 15 min. Lysate proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto PVDF membrane. The membrane was incubated with a solution containing Tris-buffered saline, 0.05% Tween-20 and 10% (w/v) non-fat dry milk, and then exposed for 1 h at room temperature to desired primary antibody. Following treatment with appropriate secondary antibody, the immunoreactive bands were visualized using enhanced chemiluminescence method. The blots were stripped and re-probed with antibodies against actin to correct for differences in protein loading.
Xenograft assay
Male or female athymic mice (6-week-old) were purchased from Charles River (Wilmington, MA), and maintained in accordance with Institutional Animal Care Use Committee guidelines. PC-3 cells were mixed in a 1:1 ratio with Matrigel (Becton Dickinson, Bedford, MA), and a 0.1 ml suspension containing 10 6 cells was injected subcutaneously on both left and right flank of each mouse. Mice were randomized into two groups of 5 mice/group (2 tumors/mouse). Experimental animals were treated orally with SFN (5.6 µmol SFN in 0.1 ml PBS) 3 times/week (Monday, Wednesday and Friday) beginning the day of tumor cell implantation. Control mice received an equal volume of the vehicle. Tumor volume was determined as described by us previously ( 33 ). Statistical significance of difference in tumor volume, wet tumor weight or body weight between control and treated mice was assessed by Student's t -test. At the termination of the experiment, the tumor tissues were harvested and divided into two pieces. A portion of the tumor tissue was processed for immunohistochemistry for analysis of apoptotic bodies, whereas the second piece was used for western blotting. For immunohistochemistry, tumor tissues were embedded in paraffin, sectioned (6 µm), de-paraffinized and processed for determination of apoptotic bodies using ApoTag Plus Peroxidase In Situ Apoptosis detection kit (Intergen, NY) according to the manufacturer's instructions. Brown color apoptotic bodies in tumor sections of control and SFN-treated mice were counted under a Nikon Eclipse E800 microscope at 20× magnification. Three randomly selected fields on each tumor section were counted for apoptotic bodies. For western blotting, tumor tissues were minced, suspended in PBS and homogenized using a polytron. The homogenate was centrifuged at 14 000 r.p.m. for 30 min. The supernatant fraction was collected and used for western blotting.
Results
SFN inhibits proliferation of PC-3 cells in culture
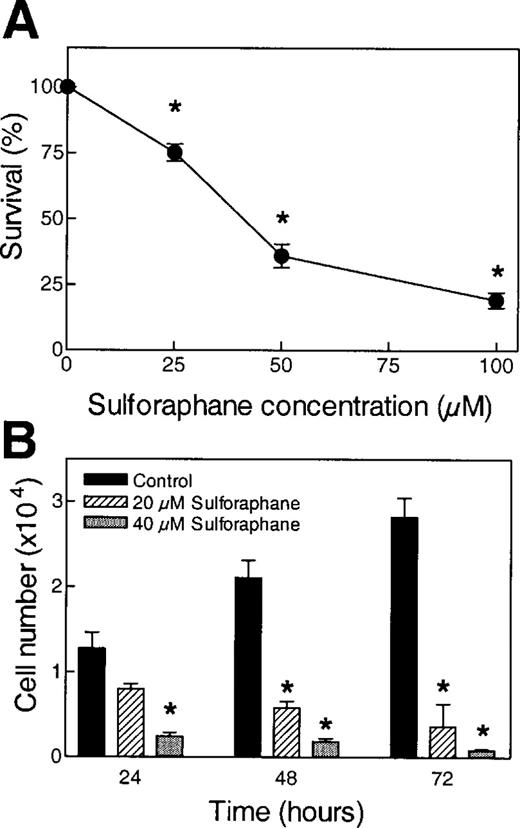
Effect of SFN on proliferation of PC-3 cells was determined by sulforhodamine B and trypan blue dye exclusion assays in two independent experiments, and the results from a representative experiment are shown in Figure 1 . Sulforhodamine B assay revealed a concentration-dependent inhibition in survival of PC-3 cells upon a 24 h exposure to SFN with an IC 50 of ∼40 µM ( Figure 1A ). In agreement with the results of sulforhodamine B assay, SFN treatment caused a statistically significant decrease in number of viable cells in trypan blue dye exclusion assay ( Figure 1B ). For example, in comparison with DMSO-treated control, the number of viable cells following a 24 h exposure to 20 and 40 µM SFN was reduced by ∼37 and 81%, respectively ( Figure 1B ). A greater effect was evident following a 48 or 72 h exposure to SFN at both the concentrations. These observations indicated that PC-3 cell line was highly sensitive to growth inhibition by SFN.
Effect of SFN treatment on proliferation of PC-3 cells determined by ( A ) sulforhodamine B assay and ( B ) trypan blue dye exclusion assay. For sulforhodamine B assay, PC-3 cells were exposed to DMSO (control) or desired concentration of SFN for 24 h. Data are mean ± SE ( n = 6) from a representative experiment that was repeated two times, and the results were comparable. For trypan blue assay, cells were treated with DMSO (control) or 20 or 40 µM SFN for 24, 48 or 72 h. Data are mean ± SE ( n = 3) from a representative experiment that was repeated two times, and the results were comparable. * Significantly different compared with control ( P < 0.05) by Student's t -test.
SFN induces apoptosis in PC-3 cells
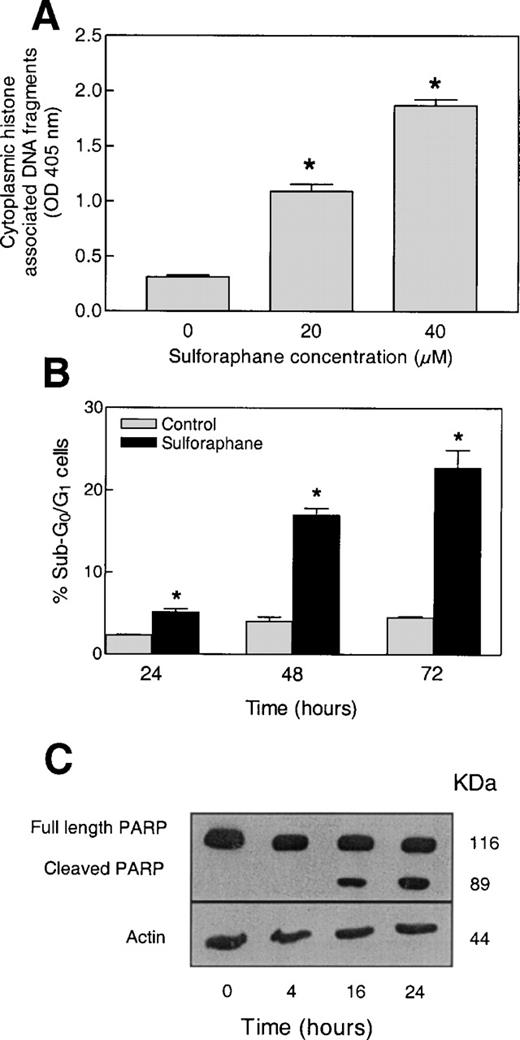
Apoptosis is a widely accepted mechanism for antiproliferative activity of many naturally occurring as well as synthetic agents. Apoptotic cell death is characterized by a number of morphological and cellular changes, including chromatin condensation, membrane blebbing, DNA fragmentation, and cleavage of key cellular proteins such as PARP. We conducted experiments to address the question of whether antiproliferative activity of SFN against PC-3 cells was due to apoptosis induction. Apoptosis inducing effect of SFN was assessed by ELISA based quantification of cytoplasmic histone associated DNA fragments. As can be seen in Figure 2A , a 24 h treatment of PC-3 cells with SFN resulted in a concentration-dependent and statistically significant increase in the levels of cytoplasmic histone associated DNA fragments when compared with control. In comparison with DMSO-treated control, treatment of cells with 20 and 40 µM SFN resulted in ∼3.5- and 6.0-fold increase in the levels of cytoplasmic histone associated DNA fragments ( Figure 2A ). Apoptosis inducing effect of SFN was confirmed by (i) flow cytometric analysis of cells with sub-G 0 /G 1 DNA content ( Figure 2B ) and (ii) western blot analysis for PARP cleavage ( Figure 2C ). Fraction of cells with sub-G 0 /G 1 DNA content was increased by ∼2.2-, 4.3- and 5.0-fold, respectively, upon exposure of PC-3 cells to 20 µM SFN for 24, 48 and 72 h ( Figure 2B ). In time course experiments using 20 µM SFN, an immunoreactive band corresponding to cleaved PARP (89 kDa) was observed at 16 and 24 h time points with a concomitant decrease in the level of full length 116 kDa PARP ( Figure 2C ). Taken together, these observations clearly indicated that antiproliferative effect of SFN against PC-3 cells was associated with apoptosis induction.
Effect of SFN treatment on apoptosis induction determined by ( A ) quantification of cytoplasmic histone associated DNA fragments following a 24 h exposure to DMSO (control) or SFN (20 or 40 µM), ( B ) flow cytometric analysis of cells with sub-G 0 /G 1 DNA content following a 24, 48 or 72 h treatment with DMSO (control) or 20 µM SFN, and ( C ) western blot analysis for PARP cleavage using lysates from PC-3 cells exposed to 20 µM SFN for different time intervals (each lane in panel C contained 40 µg of lysate protein). Experiments for data in (A), (B) and (C) were repeated two times, and the results were comparable. Data from a representative experiment are shown, and mean ± SE of three determinations (A and B). * Significantly different compared with control ( P < 0.05) by Student's t -test.
SFN-induced apoptosis in PC-3 cells is associated with Bax over-expression and Bcl-2 down-regulation
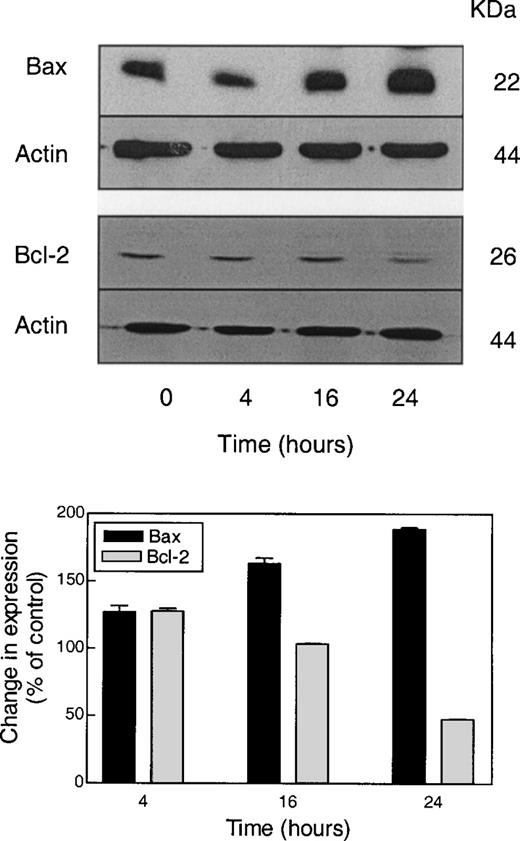
The effect of SFN treatment on expression of Bcl-2 family of anti- (Bcl-2 and Bcl-X L ) and pro-apoptotic (Bax and BID) proteins was determined to gain insights into the mechanism for SFN-induced cell death. Representative western blots depicting effect of SFN treatment on expression of Bax and Bcl-2 proteins are shown in Figure 3 . Exposure of PC-3 cells to 20 µM SFN resulted in up-regulation of Bax expression that was evident at 16 and 24 h time points ( Figure 3A and B ). At the same time, a marked decrease in Bcl-2 expression was observed in SFN-treated cells at 24 h (53% reduction compared with control) time point ( Figure 3A and B ). SFN treatment did not reduce expression of Bcl-X L or BID (data not shown). These observations indicated that SFN-induced apoptosis might be manifested by an increase in Bax : Bcl-2 ratio.
Representative western blots for expression of Bax and Bcl-2 using lysates from PC-3 cells exposed to 20 µM SFN for specified time intervals. Equal amounts of lysate protein (20 µg for Bax and 40 µg for Bcl-2) were subjected to gel electrophoresis. Blots were stripped and re-probed with antibodies against actin to correct for differences in protein loading. The bar diagram summarizes changes in Bax and Bcl-2 expression relative to control. Data are mean of two to three independent experiments.
Involvement of caspases in SFN-induced apoptosis
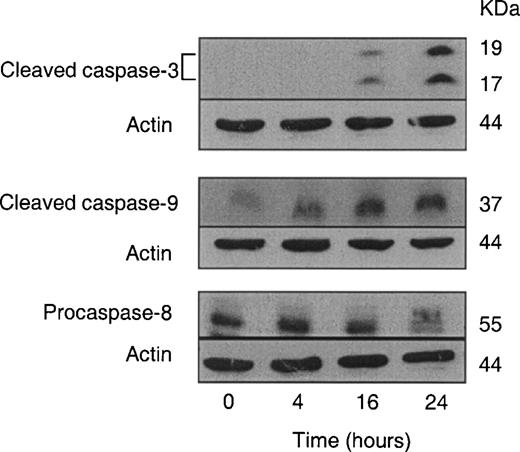
Caspases are aspartate-specific cysteine proteases that play critical roles in apoptosis ( 34 – 37 ). Activation of caspases results in cleavage and inactivation of key cellular proteins, including the DNA repair enzyme PARP ( 35 ). Since PARP cleavage was observed in SFN-treated PC-3 cells ( Figure 2C ), we reasoned that SFN-induced apoptosis might involve caspases. We explored this possibility by determining the effect of SFN treatment on activation of caspases by western blotting using antibodies that recognize either full-length (procaspase-8) or cleaved (caspase-3 and caspase-9) caspases, and representative blots are shown in Figure 4 . Treatment of PC-3 cells with 20 µM SFN resulted in cleavage of procaspase-3 as evidenced by appearance of 19 and 17 kDa intermediates at 16 and 24 h time points. Caspase-3 is an executioner caspase that can be activated by (i) a mitochondrial pathway involving activation of caspase-9 due to release of cytochrome c to the cytosol, which leads to its binding to apoptosis protease activation factor-1 and subsequent recruitment and activation of procaspase-9 or (ii) a death receptor pathway involving caspase-8 ( 34 – 39 ). Alternatively, active caspase-8 can either directly cleave procaspase-3 or it can cleave pro-apoptotic protein BID and truncated BID translocates to the mitochondria and induces release of cytochrome c ( 34 , 40 ). As shown in Figure 4 , treatment of PC-3 cells with 20 µM SFN resulted in appearance of 37 kDa procaspase-9 cleavage intermediate, which preceded caspase-3 cleavage and was evident as early as 4 h after SFN treatment. On the other hand, cleavage of caspase-3 was not observed until 16 h. Western blotting using antibodies specific for procaspase-8 revealed an ∼60% decrease in its level at 24 h time point ( Figure 4 ). These observations pointed towards involvement of both caspase-9 and caspase-8 in SFN-mediated cleavage of caspase-3.
Western blot analysis for cleaved caspase-3, cleaved caspase-9 and procaspase-8 using lysates from PC-3 cells exposed to 20 µM SFN for specified time intervals. Equal amounts of lysate protein (80 µg for cleaved caspase-3, and 60 µg for cleaved caspase-9 and procaspase-8) were subjected to gel electrophoresis. Data are representative of at least two independent experiments with similar results.
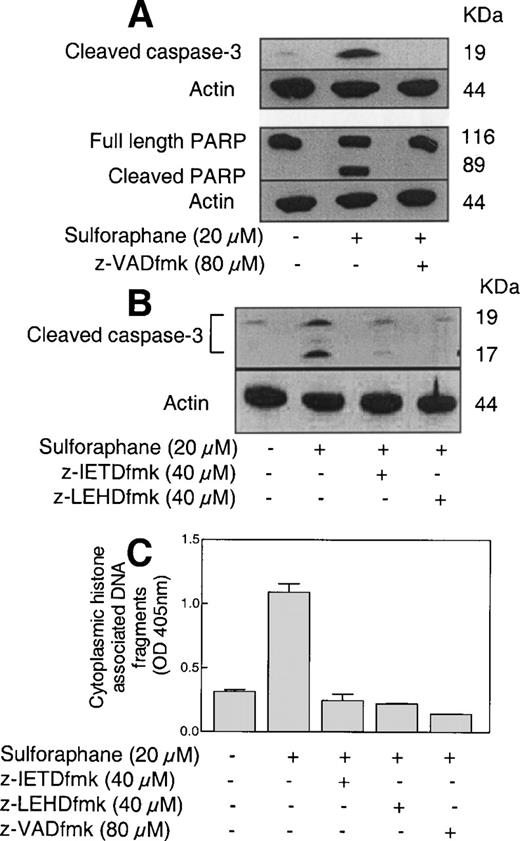
Reversal of SFN-induced apoptosis by caspase inhibitors
To determine relative contribution of caspase-9 and caspase-8 pathways to SFN-induced apoptosis, the effects of general caspase inhibitor z-VADfmk, caspase-8 specific inhibitor z-IETDfmk, and caspase-9 specific inhibitor z-LEHDfmk on SFN-induced cleavage of procaspase-3 and PARP, and apoptosis were determined. As can be seen in Figure 5A , treatment of PC-3 cells with 20 µM SFN for 24 h resulted in cleavage of procaspase-3 as well as PARP, which was fully blocked upon a 2 h pre-treatment of cells with 80 µM z-VADfmk (general caspase inhibitor). For reasons not clear, the 17 kDa procaspase-3 intermediate was not detected in this immunoblotting experiment. As can be seen in Figure 5B , SFN-induced cleavage of procaspase-3 was partially abolished in the presence of caspase-8 specific inhibitor z-IETDfmk and caspase-9 specific inhibitor z-LEHDfmk, which is reflected by a decrease in intensity of bands corresponding to 19 and 17 kDa intermediates. However, a relatively greater reversal of procaspase-3 cleavage was observed for caspase-9 inhibitor than that for caspase-8-specific inhibitor. SFN-induced cleavage of PARP was also blocked by pre-treatment of cells with caspase-8 and caspase-9 inhibitors (data not shown in Figure 5B ). As can be seen in Figure 5C , SFN-induced formation of cytoplasmic histone associated DNA fragments, a measure of apoptotic cell death, was prevented upon pre-treatment of cells with z-VADfmk, z-IETDfmk and z-LEHDfmk. Taken together, these results pointed towards involvement of both caspase-8 and caspase-9 pathways in execution of SFN-induced activation of caspase-3 and apoptosis.
Western blot analysis for the effects of ( A ) general caspase inhibitor z-VADfmk and ( B ) z-IETDfmk (casapsae-8-specific inhibitor) and z-LEHDfmk (caspase-9-specific inhibitor) on SFN-induced cleavage of procaspase-3 or PARP. PC-3 cells were exposed to DMSO (control) or desired caspase inhibitor for 2 h prior to treatment with 20 µM SFN for 24 h. Western blotting for the effects of caspase inhibitors on cleavage of caspase-3 and PARP were repeated two to three times using independently prepared lysates, and the results were comparable. ( C ) Effects of caspase inhibitors on SFN-induced generation of cytoplasmic histone associated DNA fragments. Data are mean ± SE ( n = 3) from a representative experiment that was repeated three times with similar results. Statistically significant difference at P = 0.05 was observed between control versus SFN alone group, and between SFN alone versus z-IETDfmk, z-LEHDfmk and z-VADfmk pre-treatment groups by ANOVA.
Orally administered SFN retards growth of PC-3 xenografts in vivo
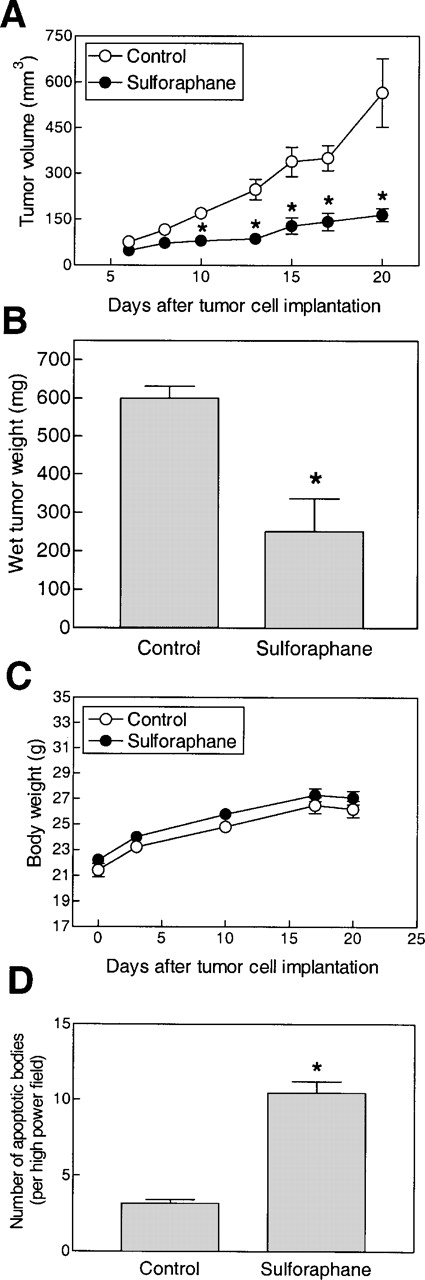
Cultured cancer cells are valuable reagents for rapid screening of potential anticancer agents as well as for elucidation of mechanism of their activity. Prior to clinical trials, however, it is essential that the in vivo efficacy of potential anticancer agents is determined in a suitable animal model. Studies were, therefore, conducted to determine whether SFN administration affects growth of PC-3 xenografts in nude mice. Effect of oral administration of SFN on growth of PC-3 xenografts was determined in two independent experiments, and data from a representative experiment are shown in Figure 6 . As can be seen in Figure 6A , SFN treatment caused a significant inhibition of PC-3 xenograft growth. For instance, 10 days after starting therapy, the average tumor volumes in control and SFN-treated mice were 170 ± 13 and 80 ± 14 mm 3 , respectively, reflecting a >50% reduction in tumor volume in SFN group. Similarly, 20 days after tumor cell implantation, the average tumor volume in SFN-treated mice (165 ± 21 mm 3 ) was ∼71% lower than that of control mice.
Effect of oral administration of SFN on ( A ) growth of PC-3 tumor xenografts in male nude mice, ( B ) wet weight of tumors harvested at the termination of the experiment, ( C ) body weight of mice and ( D ) apoptotic bodies by TUNEL assay. Data are mean ± SE. * Significantly different compared with control, P < 0.05 by Student's t -test.
Statistically significant inhibition of PC-3 tumor xenograft growth upon oral administration of SFN was also observed in the second independent experiment using female nude mice. For instance, 17 days after tumor cell implantation, the average tumor volumes in control and SFN-treated mice were 207 ± 35 and 90 ± 22 mm 3 , respectively, reflecting a >50% reduction in tumor volume in SFN-treated group.
As can be seen in Figure 6B , the average wet weight of the tumors from control mice was higher by ∼2.4-fold compared with SFN-treated mice. Body weights of the control and treated mice were recorded to determine if SFN administration causes weight loss. Average body weights of the control and SFN-treated mice did not differ significantly throughout the treatment protocol in both experiments ( Figure 6C ). Consistent with the results in cultured PC-3 cells, TUNEL assay revealed a significantly higher count of apoptotic bodies in tumor sections from SFN-treated mice compared with controls (∼3.3-fold higher compared with control, P < 0.05 by Student's t -test) ( Figure 6D ).
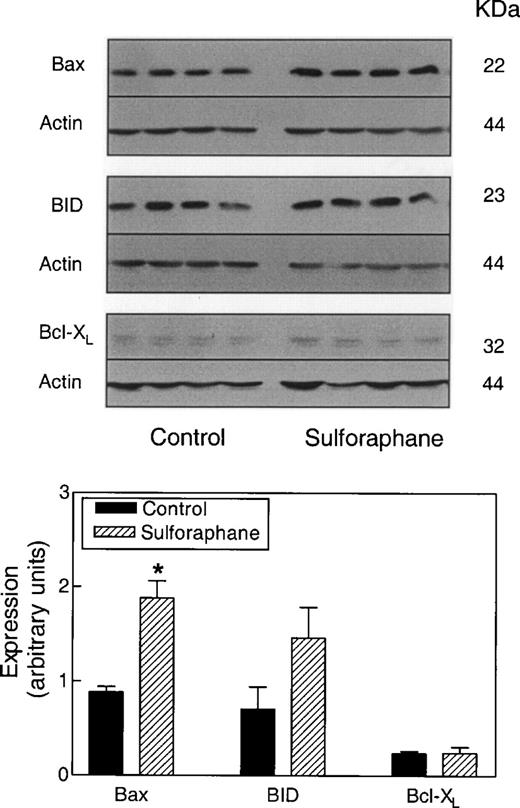
To gain insights into the mechanism for increased apoptosis, expression of Bcl-2 family of proteins (Bcl-2, Bcl-X L , Bax, BID) in tumors of control and SFN-treated mice were compared by western blotting, and the data are shown in Figure 7 . In agreement with data in cultured PC-3 cells, Bax expression was found to be significantly higher in tumors of SFN-treated mice than that of control mice (2.1-fold higher compared with control, P < 0.05 by Student's t -test). Even though, the expression of BID was slightly higher in tumors of SFN-treated mice compared with controls, the difference did not reach statistical significance ( Figure 7 ). Consistent with the results in cultured PC-3 cells, SFN administration did not reduce Bcl-X L expression. Interestingly, expression of Bcl-2 could not be detected in tumors of either control or SFN-treated mice (data not shown).
Western blot analysis for expression of Bax, BID and Bcl-X L using lysates from tumors of control and SFN-treated mice. Blots were stripped and re-probed with antibodies against actin to correct for differences in protein loading. Tumor lysates from four individual mouse of each group were used for western blotting, and data are mean ± SE. * Significantly different compared with control, P < 0.05 by Student's t -test.
Discussion
Previous studies have revealed that SFN is a potent inducer of the expression of enzymes implicated in detoxification of a variety of chemical carcinogens, and a highly effective inhibitor of chemically induced cancer in animals ( 15 – 17 , 20 ). Data presented herein indicate that SFN is highly effective in suppressing proliferation of PC-3 human prostate cancer cells in culture. In two independent experiments, we also found that oral administration of SFN (5.6 µmol SFN, 3 times/week) significantly retards growth of PC-3 xenografts in nude mice without causing weight loss. To the best of our knowledge, the present study is the first published report to document growth inhibitory effect of SFN in vivo in a tumor xenograft model. SFN-mediated growth inhibition of PC-3 cells in culture and PC-3 xenografts in vivo was observed at micromolar concentrations. Even though it is difficult to predict whether micromolar concentrations of SFN are achievable in humans as pharmacokinetics of this agent has not been investigated, previous studies have estimated that 100 g of broccoli could yield up to 40 µmol of SFN ( 41 – 44 ). A more recent pharmacokinetics study involving four human volunteers receiving single dose of ∼200 µmol of broccoli sprout indicated that ITCs were absorbed rapidly and reached peak concentrations of 0.943–2.27 µmol/l in plasma, serum and erythrocytes 1 h after ingestion of broccoli extract ( 45 ). However, carefully designed pharmacokinetic studies using pure SFN are needed to determine its achievable concentration.
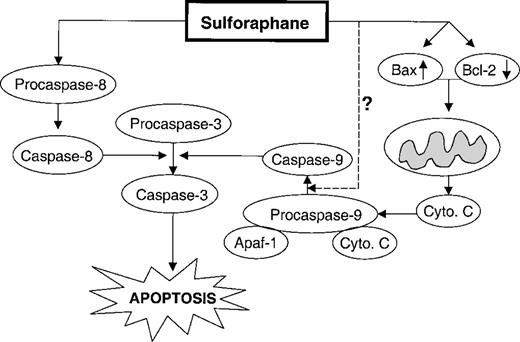
The results of the present study indicate that antiproliferative activity of SFN against PC-3 cells is due to its ability to induce apoptosis. Based on the results of the present study, mechanism for SFN-induced apoptosis in PC-3 cells is proposed in Figure 8 . We found that SFN-induced apoptosis is associated with an increase in Bax:Bcl-2 ratio resulting from up-regulation of Bax expression and down-regulation of Bcl-2 expression. Bax up-regulation has been suggested to contribute to SFN-induced apoptosis in Jurkat T-leukemia cells ( 30 ) and HT29 human colon cancer cells ( 27 ). While Bcl-2 expression could not be detected in HT29 cells ( 27 ), a slight reduction in Bcl-2 expression was observed in Jurkat cells exposed to 30 µM SFN for 48 h ( 30 ). Studies have also suggested that SFN-induced apoptosis might involve p53 as its expression was increased by ∼7-fold upon a 48 h treatment of Jurkat cells with 30 µM SFN ( 30 ). Essential role for p53 in apoptosis induction by phenethyl isothiocyanate, a structural analog of SFN, was also suggested previously ( 46 ). Our data suggest that p53 may not be required for SFN-induced apoptosis in PC-3 cells, which lack functional p53 ( 47 ).
Proposed mechanism for SFN-induced apoptosis in PC-3 cells based on the results of present study. Apoptosis induction in SFN-treated PC-3 cells and in tumors of SFN-treated mice was associated with a statistically significant increase in Bax expression but independent of a change in Bcl-X L expression. A reduction in the protein level of Bcl-2 was also observed in SFN-treated PC-3 cells. Our data using caspase inhibitors suggested involvement of caspase-9 and caspase-8 pathways in SFN-induced cleavage of procaspase-3 and PARP, and apoptosis. Dashed line indicates that the mechanism for SFN-induced cleavage of procaspase-9 at earlier time points (e.g. 4 h post-treatment) remains to be elucidated.
Activation of caspases leads to cleavage and inactivation of key cellular proteins such as PARP. In our model, PARP cleavage was observed within 16 h of SFN treatment ( Figure 2C ), which was accompanied by appearance of 17 and 19 kDa procaspase-3 cleavage intermediates ( Figure 4 ) suggesting activation of caspase-3. Caspase-3 is an executioner caspase that can be activated by a mitochondrial pathway involving release of cytochrome c ( 34 , 35 ). Alternatively, caspase-3 can be activated by caspase-8 ( 34 , 37 – 40 ). The results of the present study indicate that SFN-induced cleavage of procaspase-3 is mediated by both caspase-9 and caspase-8 pathway as cleavage of procaspase-3 and PARP in SFN-treated cells were reduced by pre-treatment of cells with pan caspase inhibitor as well as specific inhibitors of caspase-9 and caspase-8.
It is important to point out that cleavage of procaspase-9 was evident as early as 4 h after SFN treatment, whereas a meaningful change in Bax or Bcl-2 expression was not noticed until 16–24 h post-treatment. These results suggest that a change in Bax:Bcl-2 ratio may have an amplifying role rather than initiating role in cleavage of procaspase-9. It is possible that SFN-induced cleavage of procaspase-9 at earlier time points is mediated by other caspases. At the same time, possible involvement of other Bcl-2 family of apoptosis regulating proteins (e.g. Bad, Bag, Bak, Bik, etc.) in SFN-induced activation of mitochondrial caspase cascade cannot be ruled out. Interestingly, a very recent study has suggested a novel mechanism involving reactive oxygen species for activation of procaspase-9 in hypoxic cell death ( 48 ). Therefore, another possible mechanism for SFN-induced procaspase-9 cleavage could involve reactive oxygen species. Even though further studies are needed to define the precise mechanism for SFN-induced cleavage of procaspase-9 at earlier time points (e.g. 4 h after SFN treatment), the results of our inhibitor studies ( Figure 5 ) clearly indicate involvement of both caspase-8 and caspase-9 in SFN-mediated cleavage of caspase-3 and PARP, and apoptosis.
In conclusion, our data indicate that PC-3 human prostate cancer cell line is highly sensitive to growth inhibition by SFN. More importantly, we demonstrate that the growth of PC-3 xenografts in vivo in nude mice is significantly inhibited upon oral administration of SFN at concentrations that can be generated through dietary intake of broccoli and other related vegetables.
To whom correspondence should be addressed Email: singhs@msx.upmc.edu
This investigation was supported in part by USPHS grants CA101753 and CA55589 (to S.V.S.), awarded by the National Cancer Institute.
References
Verhoeven,D.T., Goldbohm,R.A., van Poppel,G., Verhagen,H. and van den Brandt,P.A. (
Kohlmeier,L. and Su,L. (
Zhang,S.M., Hunter,D.J., Rosner,B.A., Giovannucci,E.L., Colditz,G.A., Speizer,F.E. and Willett,W.C. (
Cohen,J.H., Kristal,A.R. and Stanford,J.L. (
Kolonel,L.N., Hankin,J.H., Whittemore,A.S. et al . (
Fahey,J.W., Zalcmann,A.T. and Talalay,P. (
Zhang,Y. and Talalay,P. (
Hecht,S.S. (
Talalay,P. and Fahey,J.W. (
Wattenberg,L.W. (
Stoner,G.D., Morrissey,D.T., Heur,Y.H., Daniel,E.M., Galati,A.J. and Wagner,S.A. (
Morse,M.A., Wang,C.X., Stoner,G.D., Mandal,S., Conran,P.B., Amin,S.G., Hecht,S.S. and Chung,F.L. (
Chung,F.L., Conaway,C.C., Rao,C.V. and Reddy,B.S. (
Yang,Y.M., Conaway,C.C., Chiao,J.W., Wang,C.X., Amin,S., Whysner,J., Dai,W., Reinhardt,J. and Chung,F.L. (
Zhang,Y., Talalay,P., Cho,C.G. and Posner,G.H. (
Zhang,Y., Kensler,T.W., Cho,C.G., Posner,G.H. and Talalay,P. (
Fahey,J.W., Zhang,Y. and Talalay,P. (
Gao,X., Dinkova-Kostova,A.T. and Talalay,P. (
Wu,L. and Juurlink,B.H. (
Fahey,J.W., Haristoy,X., Dolan,P.M., Kensler,T.W., Scholtus,I., Stephenson,K.K., Talalay,P. and Lozniewski,A. (
Smith,T.J., Guo,Z., Li,C., Ning,S.M., Thomas,P.E. and Yang,C.S. (
Barcelo,S., Gardiner,J.M., Gescher,A. and Chipman,J.K. (
Maheo,K., Morel,F., Langouet,S., Kramer,H., Le Ferrec,E., Ketterer,B. and Guillouzo,A. (
Ye,L. and Zhang,Y. (
Brooks,J.D., Paton,V.G. and Vidanes,G. (
Thimmulappa,R.K., Mai,K.H., Srisuma,S., Kensler,T.W., Yamamoto,M. and Biswal,S. (
Gamet-Payrastre,L., Li,P., Lumeau,S., Cassar,G., Dupont,M.A., Chevolleau,S., Gasc,N., Tulliez,J. and Terce,F. (
Bonnesen,C., Eggleston,I.M. and Hayes,J.D. (
Chiao,J.W., Chung,F.L., Kancherla,R., Ahmed,T., Mittelman,A. and Conaway,C.C. (
Fimognari,C., Nüsse,M., Cesari,R., Iori,R., Cantelli-Forti,G. and Hrelia,P. (
Xiao,D., Srivastava,S.K., Lew,K.L., Zeng,Y., Hershberger,P., Johnson,C.S., Trump,D.L. and Singh,S.V. (
Xiao,D. and Singh,S.V. (
Singh,S.V., Mohan,R.R., Agarwal,R. et al . (
Wolf,B.B. and Green,D.R. (
Budihardjo,I., Oliver,H., Lutter,M., Luo,X. and Wang,X. (
Ashkenazi,A. and Dixit,V.M. (
Kischkel,F.C., Hellbardt,S., Behrmann,I., Germer,M., Pawlita,M., Krammer,P.H. and Peter,M.E. (
Li,H., Zhu,H., Xu,C.J. and Yuan,J. (
Jiao,D., Yu,M., Hankin,J.H., Low,S.H. and Chung,F.L. (
Chiang,W.C.K., Pusateri,D.J. and Leitz,R.E.A. (
Shapiro,T.A., Fahey,J.W., Wade,K.L., Stephenson,K.K. and Talalay,P. (
Conaway,C.C., Getahun,S.M., Liebes,L.L., Pusateri,D.J., Topham,D.K., Botero-Omary,M. and Chung,F.L. (
Ye,L., Dinkova-Kostova,A.T., Wade,K.L., Zhang,Y., Shapiro,T.A. and Talalay,P. (
Huang,C., Ma,W.Y., Li,J., Hecht,S.S. and Dong,Z. (
Author notes
Department of Pharmacology, 1Department of Pathology, and University of Pittsburgh Cancer Institute, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213 and 2Hillman Cancer Center, Research Pavilion Suite 2.32A, 5117 Center Avenue, Pittsburgh, PA 15213, USA