-
PDF
- Split View
-
Views
-
Cite
Cite
My N. Chau, Lara H. El Touny, Shankar Jagadeesh, Partha P. Banerjee, Physiologically achievable concentrations of genistein enhance telomerase activity in prostate cancer cells via the activation of STAT3, Carcinogenesis, Volume 28, Issue 11, November 2007, Pages 2282–2290, https://doi.org/10.1093/carcin/bgm148
Close - Share Icon Share
Abstract
Telomerase contributes to the infinite replicative potential of cancer cells by conferring proliferation and survival through the regulation of growth factors and apoptotic proteins. Although it is generally known that the phytoestrogen, genistein, has telomerase-repressing and anti-proliferative effects on various cancer cells at pharmacological concentrations, we report here that physiologically achievable concentrations of genistein enhance telomerase activity, the proliferation of human prostate cancer cells and tumor growth in the transgenic adenocarcinoma mouse prostate model. In determining the mechanism for enhanced telomerase activity, we observed that physiological concentrations of genistein activated signal transducers and activators of transcription 3 (STAT3) both in vitro and in vivo and increased STAT3 binding to the telomerase reverse transcriptase promoter in human prostate cancer cells. These results demonstrate for the first time that physiologically achievable concentrations of genistein enhance telomerase reverse transcriptase transcriptional activity in prostate cancer cells via the activation of STAT3. Consequently, these concentrations of genistein will augment the growth of prostate cancer cells that could be detrimental to individuals with prostate cancer and therefore, caution should be exercised when genistein is considered for chemotherapeutic purposes.
Introduction
Prostate cancer is the second leading cause of cancer mortality in men in the USA ( 1 ). Epidemiological studies have suggested that phytoestrogens in soybeans can reduce the risk for the acquisition of hormone-dependent cancers ( 2 , 3 ). Genistein (4,5,7-trihydroxyisoflavone), the most abundant isoflavonoid of soybeans, has been proposed as one of the compounds underlying the reputed anticancer effect of soy ( 4 ). Several studies have reported that genistein suppresses prostate cancer cell proliferation in vitro and in vivo ( 4–6 ) possibly by inhibiting angiogenesis, tyrosine kinases, topoisomerase II, prostaglandin synthesis and telomerase activity ( 7–11 ). However, all these studies used pharmacological concentrations of genistein (>10 μM). The effect of physiological concentrations of genistein (<2 μM) in prostate cancer cells and, in particular, on telomerase activity has not been reported.
Telomerase is a ribonucleoprotein enzyme with reverse transcriptase (RT) activity that is responsible for the extension of telomeric DNA ( 12 , 13 ). The telomerase complex is composed of telomerase reverse transcriptase (TERT), telomerase RNA (TERC), telomerase-associated protein 1 (TEP1) and chaperone proteins (p23, Hsp90) ( 14 ). Telomerase is present in germ line cells, cancer-derived cell lines and spontaneously immortalized cells in culture ( 15 ), but the enzyme is usually not expressed in normal somatic cells that results in the progressive loss of telomeres with each cell division ( 15 ). Recent literature indicates that telomerase has additional functions to stabilizing chromosome ends ( 16 ). Telomerase can enhance cancer cell proliferation by increasing the transcription of growth-controlling genes and promote survival by abating apoptosis and facilitating DNA repair ( 16–18 ).
Telomerase activity is highly correlated to the expression of human telomerase reverse transcriptase (hTERT) messenger RNA (mRNA) ( 19 ) and numerous transcription factors regulate hTERT promoter activity that includes c-Myc, SP1, NFkB, MZF2, E2F-1, Ets and AP1 ( 20–23 ). In addition, a recent study demonstrated that the hTERT promoter has signal transducers and activators of transcription 3 (STAT3)-binding sites and STAT3 can bind to the hTERT promoter ( 24 ). STATs are a class of transcription factors composed of STAT 1, 2, 3, 4, 5a, 5b and 6 ( 25 ). These proteins are involved in proliferation, differentiation, apoptosis, cell survival, inflammation and immune response in normal cells ( 26 , 27 ). STAT3 is over-expressed in prostate, breast, head and neck, and blood cancers ( 28 ). It has been shown that the inhibition of STAT3 with dominant-negative (DN) constructs and small interfering RNA (siRNA) suppresses prostate cancer growth in vitro and in vivo , which implicates STAT3 as an important anticancer target ( 28 , 29 ).
We recently demonstrated that pharmacological concentrations of genistein (10–100 μM) inhibited telomerase activity and the proliferation of DU-145 and PC3 human prostate cancer cells ( 11 ). However, the effect of physiologically achievable concentrations of genistein on telomerase activity has not been investigated. In this study, we demonstrate that physiologically achievable concentrations of genistein (∼0.5–1 μM) enhance telomerase activity, the proliferation of human prostate cancer cells and prostate tumor growth in genistein-fed (250 mg/kg diet) transgenic adenocarcinoma mouse prostate (TRAMP) mice with already established prostatic intraepithelial neoplasia (PIN). The activation of STAT3 is one of the possible mechanisms for enhanced telomerase activity by genistein.
Materials and methods
Cell culture
DU-145 and LNCaP prostate cancer cells, MCF-7 breast cancer cells and SKOV-3 ovarian cancer cells were purchased from the American Type Cell Culture Collection (Manassas, VA). Cells were grown in complete growth medium (Improved Minimum Essential Medium (IMEM) without phenol red; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Quality Biologicals, Gaithersburg, MD), 2 mM glutamine, 100 units/ml penicillin G sodium and 100 μg/ml streptomycin sulfate (Sigma Chemicals, St Louis, MO) in the presence of 5% CO 2 at 37°C. The media of cells treated with genistein (Sigma Chemicals) were replenished every 24 h.
Western blot analysis and immunoprecipitation
Protein lysates were prepared from cancer cell lines and dorsolateral prostates (DLPs) of TRAMP mice that were treated with various concentrations of genistein (0–50 μM) for 1–7 days or fed a genistein-supplemented diet, respectively, according to our previous published methods ( 30 ). The lysates (50 μg) were resolved on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. The membranes were probed with antibodies for total STAT3, pSTAT3 Y705, pSTAT3 S727 (1:1000; Cell Signaling Technology, Danvers, MA), cyclin D1 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), c-Myc (1:200; Santa Cruz Biotechnology), Hsp90 (1:1000; Santa Cruz Biotechnology), hTERT (1:500; Rockland, Gilbertsville, PA), phosphoserine (1:1000; Zymed Laboratories, San Francisco, CA) and β-actin (1:10 000; Sigma Chemicals) separately. Images of the membranes were captured using a Fuji LAS-1000 Imager (Tokyo, Japan) and imported into Adobe Photoshop. Band intensities were quantified by utilizing ImageJ software (National Institutes of Health, Bethesda, MD). The preparation of nuclear extracts and immunoprecipitations were performed according to our previously published methods ( 11 ).
RT–polymerase chain reaction
RNA was extracted from DU-145 cells with TRIzol solution as suggested by the manufacturer (Invitrogen). Total RNA (500 ng) was reverse transcribed to cDNA using a Superscript II kit (Invitrogen) with random hexamers. Human-specific primers were designed using the Primer Quest program and purchased from Integrated DNA Technologies (Coralville, IA). hTERT, TEP1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primer sequences have been previously published ( 11 ). The primer sequences and polymerase chain reaction (PCR) conditions for TERC (140 bp) have been described by Yokoi et al. ( 31 ). The primer sequences for STAT3 are forward 5′-TGC CTG GAG ACA GTT GAT GTG TCA-3′ and reverse 5′-TGG AAT TTG AAT GCA GTG GCC AGG-3′ (414 bp). PCRs for STAT3 were initiated at 94°C for 2 min, followed by 28 cycles of 94°C for 1 min, 60°C for 1 min, 72°C for 1 min and final extension at 72°C for 5 min. After amplification, PCR products were separated on 1.5% agarose gels and visualized by ethidium bromide fluorescence using the Fuji LAS-1000 Imager. Images were imported to Adobe Photoshop. Band intensities were quantified by using ImageJ software.
hTERT promoter activity and STAT3 luciferase reporter assays
DU-145 cells were transfected with a full-length STAT3 promoter-luciferase construct ( 32 ) or a STAT3 luciferase reporter construct (Clontech, Mountain View, CA) (200 ng of plasmid DNA) using GeneJammer transfection reagent (Stratagene, LaJolla, CA). DU-145 cells were also co-transfected with a 3.3 kb hTERT promoter-luciferase plasmid ( 22 ) and either wild type (WT)-STAT3 ( 33 ), constitutively active (CA)-STAT3 ( 34 ) or DN-STAT3 ( 35 ) constructs. MCF-7 and SKOV-3 cells were transfected with the 3.3 kb hTERT promoter plasmid or STAT3 luciferase reporter construct. Two days after the transfection, the cells were treated with 1 and 50 μM of genistein for another 2 days. Luciferase activity was measured in cell lysates by a microplate luminometer using the Dual Luciferase Assay kit (Promega, Madison, WI) according to the manufacturer's protocol. Luciferase activity was normalized to Renilla luciferase activity by co-transfection of pRL-TK plasmid (20 ng).
Telomeric repeat amplification protocol assay
Protein lysates from DU-145 and LNCaP cells that were treated with 0–50 μM of genistein for 3 days were extracted as described by Banerjee et al. ( 30 ) and the telomeric repeat amplification protocol (TRAP) assay was performed according to the method of Kim et al. ( 15 ) with some modifications that are detailed in Jagadeesh et al. ( 11 ). A linear range of protein concentrations were used to determine the optimal amount of protein (1 μg) for the TRAP assays. Additional TRAP assays were performed after transfecting DU-145 cells with 100 nM of STAT3 siRNA or scrambled siRNA (Dharmacon, Lafayette, CO) for 3 days. Protein lysates from the DLPs of TRAMP mice fed 0, 250 and 1000 mg/kg diets of genistein were also analyzed by TRAP assays.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation assays were performed according to the manufacturer's protocol (Active Motif, Carlsbad, CA). Briefly, DU-145 cells treated with 0, 1 and 50 μM of genistein for 1 day from three confluent 100 mm plates were fixed and then lysed. The nuclear fractions were isolated, enzymatically sheared and then incubated with pSTAT3 Y705 antibody (1:50). Eluted DNA was analyzed by PCR using primers previously published by Konnikova et al. ( 24 ). PCRs were initiated at 94°C for 3 min, followed by 32 cycles of 94°C for 20 s, 58°C for 30 s, 72°C for 30 s and final extension at 72°C for 5 min.
Cell growth assay
DU-145 and prostate epithelial cells (PrECs) were seeded in 24-well plates (1 × 10 4 cells per well) in triplicate. The cells were treated with 0, 0.05, 0.5, 1 and 50 μM genistein for 3 days. Then the cells were washed with 1× phosphate-buffered saline, trypsinized and resuspended in IMEM. Trypan blue (0.4%, Sigma Chemicals) was added to the suspended cells. Both living and dead cells were counted using a hemocytometer.
Animal handling, treatment and tissue preparation
TRAMP (The Jackson Laboratory, Bar Harbor, ME) and FVB mice (Charles River Laboratories, Wilmington, MA) were maintained in the Georgetown University animal facilities in accordance with established guidelines and protocols approved by the Georgetown University Animal Care and Use Committee. Male and female TRAMP mice were mated with FVB counterparts, and heterozygous male offspring were confirmed by genotyping as described previously ( 36 ). Transgenic males (12 weeks old) (20 mice per treatment group for a total sample number of 60 mice) were fed phytoestrogen-free purified AIN-76A pellets (Harlan Teklad, Indianapolis, IN) supplemented with 0, 250 and 1000 mg/kg of genistein, until 20 weeks of age. At the end of the treatment regimens, the mice were killed and their prostates were excised, weighed and stored at −80 ° C for TRAP assays and immunoblot analyses. Histopathological evaluation was confirmed by a pathologist at Georgetown University.
Statistical analysis
All data were derived from at least three independent experiments and statistical analyses were conducted using Prism 3 GraphPad software. Values were presented as mean ± SEM. Significance level was calculated using the one-way analysis of variance followed by the Dunnett post-test with an assigned confidence interval of 95%. P value < 0.05 was considered significant.
Results
Physiological concentrations of genistein enhance telomerase activity
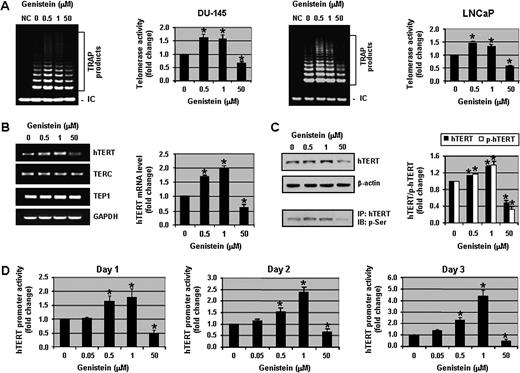
Pharmacological concentrations (10–100 μM) of genistein repress telomerase activity in human prostate cancer cells ( 11 ); therefore, 50 μM of genistein was used to compare the effects of physiologically achievable concentrations of genistein (0.5–1 μM) on telomerase activity. Genistein at 0.5 and 1 μM significantly ( P < 0.01) enhanced telomerase activity in DU-145 cells as measured by TRAP assays ( Figure 1A , left panel). The genistein-induced increase in telomerase activity was not cell line specific because a similar effect was observed in another human prostate cancer cell line, LNCaP ( Figure 1A , right panel). Quantitatively, 0.5 and 1 μM of genistein increased telomerase activity ∼1.5-fold in both cell lines. However, 50 μM of genistein repressed telomerase activity by ∼2-fold ( Figure 1A ). Although telomerase activity was increased with 0.5 and 1 μM of genistein, telomere length in DU-145 cells did not change with 1 μM of genistein treatment compared with control DU-145 cells (data not shown), suggesting that telomere length was unaffected by genistein treatment within this experimental time frame.
Physiological concentrations of genistein enhance telomerase activity in human prostate cancer cells. ( A ) Assessment of telomerase activity in DU-145 (left) and LNCaP (right) cells. Cell pellets were collected and lysates were used for TRAP assays. NC: negative control using lysis buffer only. ( B ) RNA was extracted from DU-145 cells and RT–PCR assays were performed to detect the levels of hTERT, TERC, TEP1 and GAPDH. ( C ) Western blot analysis of hTERT and phospho-hTERT protein levels in DU-145 cells. For the detection of phospho-hTERT, nuclear extracts were immunoprecipitated with hTERT antibody and immunoblotted with phosphoserine antibody. For all the experiments above, DU-145 and LNCaP cells were treated with 0, 0.5, 1 and 50 μM of genistein for 3 days. ( D ) DU-145 cells were transfected with full-length hTERT promoter-luciferase plasmid (pGL3-3328-Luc) and Renilla luciferase (pRL-TK-Luc) plasmid for 2 days in normal growth media and then exposed to various concentrations of genistein (0, 0.05, 0.5, 1 and 50 μM) for 1, 2 and 3 days. Cells were harvested and then hTERT promoter activity was determined. hTERT promoter activity was normalized to Renilla luciferase activity. Promoter activity in control samples was considered as 1.0. Columns, mean of three independent experiments; bars, standard error. * P < 0.01, significantly different from control.
Since telomerase activity is tightly correlated with the level of hTERT message, we examined the levels of hTERT, TERC and TEP1 mRNA in DU-145 cells by RT–PCR. The expression of hTERT was increased by 1.7- to 2-fold with 0.5 and 1 μM of genistein, whereas 50 μM of genistein decreased hTERT message by nearly 2-fold ( Figure 1B ). The levels of TERC and TEP1 mRNA were unchanged with genistein treatment, suggesting that the effects of genistein were hTERT specific. To ensure that the increased hTERT message translated to increased hTERT protein, hTERT protein levels were examined by western blot analysis. A dose-dependent increase in hTERT protein levels was observed and 1 μM of genistein increased hTERT levels by ∼1.5-fold. Moreover, phosphorylation of hTERT at serine residues was also increased significantly ( P < 0.01) with 0.5 and 1 μM of genistein. As expected, 50 μM of genistein induced the down-regulation of hTERT and phospho-hTERT ( Figure 1C ). Therefore, the increase in telomerase activity with 0.5 and 1 μM of genistein was substantiated by an increase in hTERT mRNA and protein expression.
To confirm the results of the TRAP assays and hTERT RT–PCR, hTERT transcriptional activity was assessed by transfecting DU-145 cells with a full-length (3.3 kb) hTERT promoter-luciferase construct ( 22 ). We observed that both 0.5 and 1 μM of genistein increased hTERT promoter activity significantly ( P < 0.01) and in a time-dependent manner (∼4-fold increase by 3 days with 1 μM genistein). The pharmacological concentration of 50 μM genistein decreased hTERT promoter activity to 50% of the control level ( Figure 1D ). Similar results were observed in MCF-7 breast cancer cells and SKOV-3 ovarian cancer cells (supplementary Figure 1A and B is available at Carcinogenesis Online). Collectively, these results show that genistein has a biphasic effect on the regulation of telomerase activity. Physiologically achievable concentrations of genistein (0.5–1 μM) enhance telomerase activity, whereas a pharmacological concentration (50 μM) represses telomerase activity in human prostate as well as in breast and ovarian cancer cells.
Physiologically achievable concentrations of genistein increase the phosphorylation of STAT3
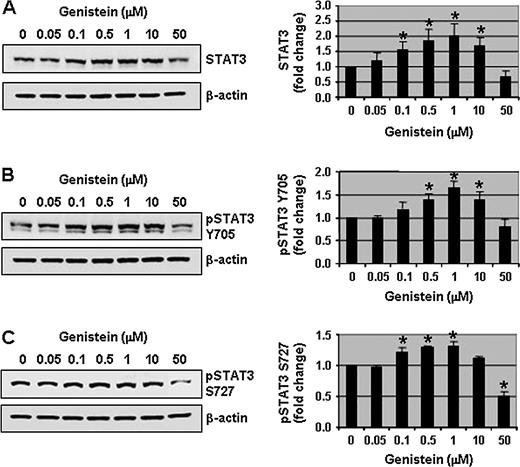
In search of various transcription factors that interact with the hTERT promoter, we chose STAT3 as a possible transcription factor because it regulates c-Myc expression and c-Myc positively regulates hTERT promoter activity. Moreover, using Genomatix software, we found several STAT-binding sites on the hTERT promoter. While we were thinking that STAT3 could be a possible regulator of the hTERT promoter, an excellent study by Konnikova et al. ( 24 ) demonstrated that the hTERT promoter has three STAT3-binding sites and STAT3 regulates hTERT expression in human glioblastoma cells. This prompted us to investigate whether physiological concentrations of genistein activate STAT3 to modulate hTERT transcriptional activity. Therefore, the effect of genistein on total and phosphorylated (activated) STAT3 was examined. We observed that 0.1–1 μM of genistein treatment for 3 days significantly ( P < 0.01) increased total STAT3 protein levels 1.5- to 2-fold in DU-145 cells ( Figure 2A ) and increased the phosphorylation of STAT3 on Y705 and S727 residues 1.5-fold above control levels ( Figure 2B and C ). This activation of STAT3 was not a transient event since the increased expression of both pSTAT3 Y705 and pSTAT3 S727 was sustained up to 7 days with 1 μM of genistein treatment (supplementary Figure 2B and C is available at Carcinogenesis Online). In contrast, a pharmacological concentration of genistein (50 μM) decreased the total and phosphorylated STAT3 levels significantly ( P < 0.01) ( Figure 2A–C ). Since 0.5 and 1 μM of genistein induced the most significant activation of STAT3, all subsequent experiments were performed using these two doses.
Physiological concentrations of genistein increase the phosphorylation of STAT3. Protein lysates (50 μg) from DU-145 cells treated with a range of genistein concentrations (0, 0.05, 0.1, 0.5, 1, 10 and 50 μM) for 3 days were resolved on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblots were probed with antibodies to total STAT3 ( A ), pSTAT3 Y705 ( B ) and pSTAT3 S727 ( C ). All immunoblots were reprobed with β-actin antibody to ensure equal loading. Representative photographs from an experiment that was repeated thrice. Quantitative analyses of relative levels of total and pSTAT3 (Y705 and S727) are shown on the right panels. Columns, mean; bars, standard error. * P < 0.01, significantly different from control.
To verify that the effect of genistein was not cell line specific, the activation of STAT3 by physiologically achievable concentrations of genistein was investigated in another prostate cancer cell line. Similar to DU-145 cells, 1 μM of genistein treatment also increased total STAT3 and pSTAT3 S727 in LNCaP cells (supplementary Figure 3A and B is available at Carcinogenesis Online). Furthermore, the activation of STAT3 by physiologically achievable concentrations of genistein was also observed in breast and ovarian cancer cells (supplementary Figure 1C and D is available at Carcinogenesis Online). These results show that physiologically achievable doses of genistein increased the activation of STAT3, and this activation is not prostate cancer cell line specific.
The increased phosphorylation of STAT3 observed by western blot analyses indicates that more active STAT3 should be present in the nucleus and therefore, we performed immunofluorescence stainings of total STAT3 and pSTAT3 Y705 in DU-145 cells treated with or without genistein for 1 day. Total STAT3 is localized predominantly in the nucleus in control DU-145 cells with some STAT3 in the cytoplasm. The nuclear localization of total STAT3 and pSTAT3 Y705 increased with 0.5 μM of genistein treatment, whereas 50 μM of genistein completely abolished nuclear staining and minimal STAT3 staining was present in the cytoplasm (supplementary Figure 4 is available at Carcinogenesis Online). These results demonstrate that physiologically achievable concentrations of genistein increased activated STAT3 in the nuclei of prostate cancer cells.
Physiological concentrations of genistein increase STAT3 transcriptional activity and STAT3-responsive proteins in human prostate cancer cells
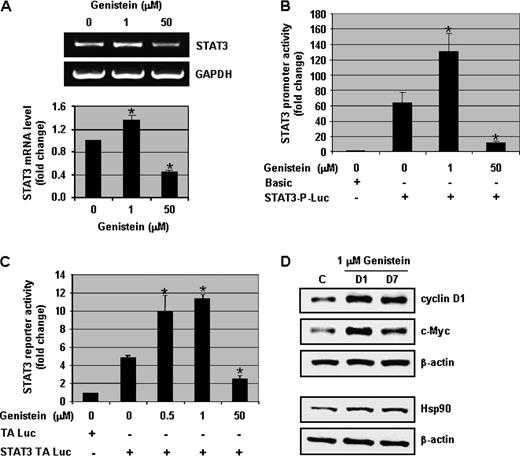
The level of STAT3 message was examined since total STAT3 protein expression increased with physiological concentrations of genistein. The STAT3 mRNA level in DU-145 cells was significantly ( P < 0.01) increased by 1.4-fold with 1 μM of genistein and decreased by 2-fold with 50 μM of genistein ( Figure 3A ). To verify the increase in STAT3 transcription, STAT3 promoter activity assays were performed. The activity of the STAT3 promoter was increased 2-fold with 1 μM of genistein treatment, whereas 50 μM of genistein decreased STAT3 transcriptional activity significantly ( P < 0.01) ( Figure 3B ). Similarly, STAT3 reporter activity increased >2-fold with 0.5 and 1 μM of genistein treatment in DU-145 cells and 50 μM of genistein decreased STAT3 transcriptional activity by ∼2-fold ( Figure 3C ). Comparable results were observed with MCF-7 and SKOV-3 cells (supplementary Figure 1E and F is available at Carcinogenesis Online).
Physiologically achievable concentrations of genistein increase the transcriptional activity of STAT3 and the expression of STAT3 target proteins. ( A ) DU-145 cells were exposed to 0, 1 and 50 μM of genistein for 3 days. RNA was extracted and RT–PCR assays were performed to assess the levels of STAT3 and GAPDH mRNA. Representative photographs from an experiment that was repeated thrice. Quantitative estimations of STAT3 mRNA were determined by densitometric measurements of RT–PCR product bands after normalization with GAPDH. ( B ) DU-145 cells were transfected with full-length STAT3 promoter-luciferase plasmid and Renilla luciferase (pRL-TK-Luc) plasmid and then exposed to 0, 1 and 50 μM of genistein for 2 days. Cells were harvested and promoter assays were performed. STAT3 promoter activity was normalized to Renilla luciferase activity. Promoter activity in control samples (with pGL3-basic) was considered as 1.0. ( C ) DU-145 cells were transfected with STAT3-TA luciferase plasmid and Renilla luciferase (pRL-TK-Luc) plasmid and then treated with different concentrations of genistein (0, 0.5, 1 and 50 μM) for 2 days. Cells were harvested and reporter assays were performed. STAT3 reporter activity was normalized to Renilla luciferase activity. Reporter activity in control samples (with TA-Luc) was considered as 1.0. ( D ) Protein lysates (50 μg) from DU-145 cells treated with 0 and 1 μM of genistein for 1 and 7 days were resolved on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblots were probed with antibodies to cyclin D1, c-Myc and Hsp90. All immunoblots were reprobed with β-actin antibody to ensure equal loading. Columns, mean of three independent experiments; bars, standard error. * P < 0.01, significantly different from control.
To confirm that genistein-induced increase in STAT3 transcriptional activity was functional, various STAT3-responsive proteins (cyclin D1, c-Myc and Hsp90) were examined by western blot analyses. Cyclin D1, c-Myc and Hsp90 protein levels were increased as early as 1 day after 1 μM of genistein treatment and sustained up to 7 days (end of our experiment) ( Figure 3D ). Similarly, cyclin D1 expression was increased by 1.4-fold with 1 μM and decreased with 50 μM of genistein in LNCaP cells (supplementary Figure 3C is available at Carcinogenesis Online). These results show that in addition to the phosphorylation of STAT3, physiologically achievable concentrations of genistein enhance the transcriptional activity of STAT3.
STAT3 regulates telomerase activity in human prostate cancer cells
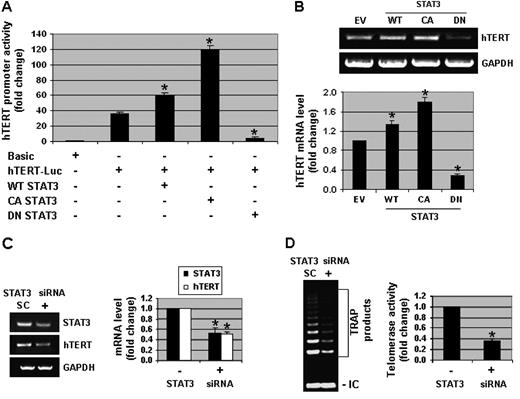
Since physiological concentrations of genistein enhanced the activation of STAT3 and telomerase activity, we were interested in examining the modulation of hTERT transcriptional activity by STAT3. Transfection of DU-145 cells with WT-STAT3 and CA-STAT3 produced a 1.5- to 3-fold increase in the transcriptional activity of the hTERT promoter ( Figure 4A ). Conversely, the inactivation of STAT3 by DN-STAT3 decreased hTERT promoter activity by 8-fold. Consistent with the results of the hTERT promoter assays, hTERT mRNA levels were increased by 1.3- and 1.8-fold with WT-STAT3 or CA-STAT3, respectively, and decreased by 3-fold with DN-STAT3 ( Figure 4B ). Similarly, transfection of STAT3 siRNA in DU-145 cells decreased the levels of STAT3 and hTERT mRNA by 2-fold ( Figure 4C ) and telomerase activity by 3-fold ( Figure 4D ). These results demonstrate that STAT3 regulates telomerase activity in human prostate cancer cells.
STAT3 modulates the transcriptional activity of hTERT. ( A ) DU-145 cells were transfected with a full-length hTERT promoter-luciferase plasmid (pGL3-3328-Luc), Renilla luciferase (pRL-TK-Luc) plasmid and either wild type (WT)-STAT3, constitutively active (CA)-STAT3 or dominant negative (DN)-STAT3 constructs. Cells were then harvested and promoter assays were performed. hTERT promoter activity was normalized to Renilla luciferase activity. Promoter activity in control samples (with pGL3-basic) was considered as 1.0. ( B ) DU-145 cells were transfected with empty vector (EV), WT-STAT3, CA-STAT3 or DN-STAT3 plasmids. RNA was extracted and RT–PCR assays were performed to measure the mRNA expression of hTERT and GAPDH. Representative photographs from an experiment that was repeated thrice. Quantitative estimations of hTERT mRNA were determined by densitometric measurements of RT–PCR product bands after normalization with GAPDH. ( C ) DU-145 cells were transfected with 100 nM of STAT3 siRNA (+) or scrambled siRNA (SC) plasmids for 3 days. RNA was isolated and RT–PCR assays were performed to detect the mRNA levels of STAT3, hTERT and GAPDH. Quantitative estimations of STAT3 and hTERT mRNA were determined by densitometric measurements of RT–PCR product bands from three independent experiments after normalization with GAPDH. ( D ) DU-145 cells were transfected with STAT3 siRNA or SC plasmids as mentioned above. Cell pellets were collected and lysates were used for TRAP assays. Quantitative estimations of telomerase activity were determined by densitometric measurements of TRAP products from three independent experiments. Columns, mean; bars, standard error. * P < 0.01, significantly different from control.
Genistein enhances STAT3 binding to the hTERT promoter and STAT3 is required for the genistein-induced increase in telomerase activity in human prostate cancer cells
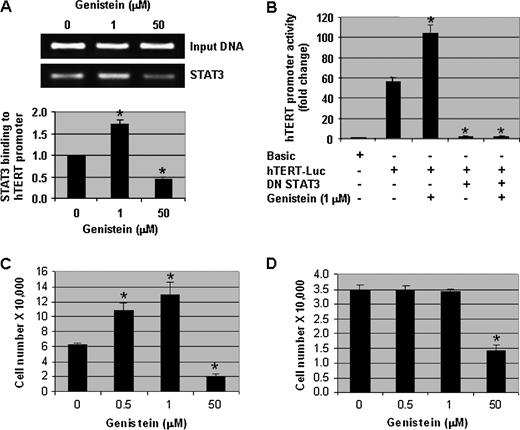
Although we demonstrated that STAT3 can regulate hTERT transcriptional activity and another laboratory reported that STAT3 can bind to the hTERT promoter ( 24 ), we have not elucidated whether physiological concentrations of genistein can increase STAT3 binding to the hTERT promoter in order to enhance hTERT promoter activity. Therefore, utilizing a chromatin immunoprecipitation assay with hTERT primers flanking the first STAT3-binding site located at −3308 bp, the physical interaction of STAT3 with the hTERT promoter was confirmed and 1 μM of genistein treatment for 1 day increased this binding 1.8-fold. In contrast, 50 μM of genistein decreased STAT3 binding to the hTERT promoter by 2-fold ( Figure 5A ). These results show that a physiologically achievable concentration of genistein enhanced the interaction of STAT3 with the hTERT promoter.
Genistein regulates telomerase activity in human prostate cancer cells via STAT3 and the increase in telomerase activity is associated with enhanced cell proliferation. ( A ) Chromatin immunoprecipitation assay showing the interaction of STAT3 with the hTERT promoter. Input DNA samples (5%) are from cell lysates prior to immunoprecipitation. Quantitative estimations are from three independent experiments. ( B ) DU-145 cells were transfected with full-length hTERT promoter-luciferase plasmid (pGL3-3328-Luc), Renilla luciferase (pRL-TK-Luc) plasmid and DN-STAT3 plasmid for 2 days in normal growth media and then treated with 0 and 1 μM of genistein for 2 days. Cells were harvested and promoter assays were performed. hTERT promoter activity was normalized to Renilla luciferase activity. Promoter activity in control samples (with pGL3-basic) was considered as 1.0. ( C ) DU-145 and ( D ) PrECs were treated with various concentrations of genistein (0, 0.5, 1 and 50 μM) for 3 days and then viable cells (as assessed by trypan blue exclusion) were counted using a hemocytometer. Columns, mean of three independent experiments; bars, standard error. * P < 0.01, significantly different from control.
To determine if STAT3 activity is necessary for the increase in hTERT transcriptional activity by physiological concentrations of genistein, DU-145 cells were transiently transfected with a full-length hTERT promoter luciferase construct along with or without DN-STAT3. The transfected cells were then treated with 0 or 1 μM of genistein for 2 days. As seen in Figure 5B , transfection of hTERT promoter alone gave ∼50-fold induction of luciferase activity. hTERT promoter activity increased another 2-fold with 1 μM of genistein. However, DN-STAT3 almost completely repressed hTERT promoter activity and 1 μM of genistein could not reverse this repression ( Figure 5B ). These results demonstrate that STAT3 activity is required for genistein-induced enhancement of hTERT transcriptional activity.
Physiologically achievable concentrations of genistein increase the growth of cancer cells
Since physiologically achievable concentrations of genistein enhanced telomerase activity and activated STAT3, the question arose whether these concentrations of genistein enhance cell growth. We observed that 0.5 and 1 μM of genistein increased the number of DU-145 cells significantly ( P < 0.01) compared with the vehicle-treated control cells. As expected, 50 μM of genistein inhibited DU-145 cell growth completely ( Figure 5C ). Complementary results were observed with BrdU labeling (data not shown). An increase in cell growth was also observed in MCF-7 and SKOV-3 cells (supplementary Figure 1G and H is available at Carcinogenesis Online). These results suggest that certain physiological concentrations of genistein enhance the growth of prostate as well as breast and ovarian cancer cells.
To evaluate whether genistein-induced cell growth only affects cancer cells that express hTERT and possess telomerase activity, normal PrECs that lack hTERT and telomerase activity (data not shown) were treated with varying concentrations of genistein and then cell growth was assessed. Physiologically achievable concentrations of genistein (0.5 and 1 μM) did not enhance the growth of PrECs ( Figure 5D ), suggesting that the presence of hTERT may be necessary for the growth-enhancing effects of genistein.
Genistein incorporation in the diet activates telomerase and STAT3 and enhances the prostate tumor size of TRAMP mice
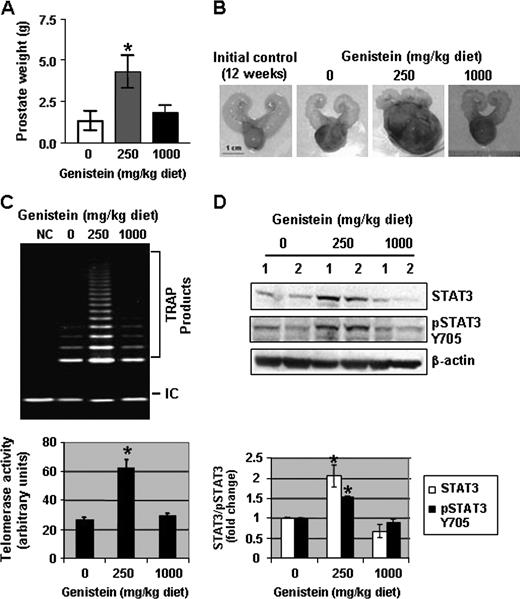
One important aspect of this study is whether the concentrations of genistein that enhance telomerase activity, activation of STAT3 and cell proliferation would have a similar effect in vivo . In order to answer this question, we characterized prostate cancer progression in TRAMP mice and observed a 100% incidence of PIN by 12 weeks of age (data not shown). Twelve-week-old TRAMP mice were then placed on AIN-76A diet regimens supplemented with 0, 250 and 1000 mg/kg of genistein until 20 weeks of age ( n = 20 mice per group), resulting in approximate serum genistein levels of 4, 350 and 3000 nM, respectively (data not shown). We observed >3-fold increase in prostate weights of TRAMP mice consuming the 250 mg/kg genistein-supplemented diet with a mean of 4.3 g ± 0.99 when compared with the prostate weights of TRAMP mice in both the 0 and 1000 mg/kg genistein diet regimens which averaged 1.28 ± 0.59 and 1.79 ± 0.474 g, respectively, suggesting a biphasic effect of genistein on TRAMP prostate tumor growth ( Figure 6A ). Representative images of prostate samples from the three treatment groups as well as a sample illustrating prostate size at the initiation of genistein treatment regimens are included in Figure 6B .
Genistein in the diet enhances tumor growth, telomerase activity and STAT3 phosphorylation. ( A ) Average prostate weight of three groups of TRAMP mice ( n = 20 per treatment group) fed an AIN-76A diet containing 0, 250, and 1000 mg/kg of genistein from 12 to 20 weeks of age. Columns, mean of three independent experiments; bars, standard error. * P < 0.05. ( B ) Illustration of representative prostates from the three treatment regimens (0, 250 and 1000 mg/kg diet) and a representative sample of prostate size at the initiation of treatment regimens (12 weeks). ( C ) Assessment of telomerase activity (as measured by TRAP assays) in DLPs of TRAMP mice placed in the three treatment groups. NC, negative control using lysis buffer only. Quantitative estimations of telomerase activity were determined by densitometric measurement of TRAP product bands from three different prostate samples. ( D ) Protein lysates (50 μg) from DLPs of two TRAMP mice in each treatment group (0, 250 and 1000 mg/kg diet) from 12 to 20 weeks of age were resolved on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblots were probed with antibodies to total STAT3 and pSTAT3 Y705. All immunoblots were reprobed with β-actin antibodies to ensure equal loading. Quantitative estimations of STAT3 and pSTAT3 Y705 were determined by densitometric measurements from three independent experiments after normalization with β-actin. Columns, mean; bars, standard error. * P < 0.01, significantly different from control.
The effect of genistein consumption on telomerase activity in the DLPs of TRAMP mice was determined. We observed a 3-fold increase in telomerase activity in the DLPs of TRAMP mice consuming the 250 mg/kg genistein supplemented diet as opposed to TRAMP mice on the control and 1000 mg/kg diets ( Figure 6C ). Furthermore, the DLPs that exhibited enhanced telomerase activity were also characterized by an up-regulation of STAT3 (2-fold) and phospho-STAT3 Y705 (1.5-fold) by western blot analysis ( Figure 6D ), demonstrating the concomitant genistein-induced activation of STAT3 and elevation of telomerase activity in DLPs of TRAMP mice. Thus, the effects of physiological concentrations of genistein in vitro were corroborated in vivo .
Discussion
It is generally believed that the phytoestrogen, genistein, has anti-proliferative effects. Recently, we and others have demonstrated that genistein has telomerase-repressing activity in human prostate cancer cells ( 11 , 37 ), which could be one mechanism for the anti-proliferative effects of genistein. However, the concentration of genistein required for achieving an anti-proliferative effect is pharmacological and cannot be obtained by consuming a phytoestrogen-containing diet. Previous studies have established that a plasma concentration of ∼0.28–2.4 μM of genistein can be achieved in humans consuming a soy-based diet ( 38 , 39 ). Therefore, we were interested in evaluating the effects of physiologically achievable concentrations of genistein on telomerase activity. To our surprise, we observed that physiologically achievable concentrations of genistein (0.5–1 μM) enhanced telomerase activity in human prostate cancer cells (DU-145 and LNCaP) and DLPs of TRAMP mice (∼0.35 μM serum concentration). It was suspected that genistein may be regulating some transcription factors to enhance telomerase activity since the transcriptional activity of the hTERT promoter was increased. The identification of STAT3-binding sites in the hTERT promoter led us to examine the effect of genistein on the expression of STAT3 in prostate cancer cells as well as in DLPs of TRAMP mice. The levels of total and phosphorylated STAT3 were up-regulated by physiological concentrations of genistein in vitro and in vivo . To our knowledge, this is the first report to demonstrate that physiologically achievable concentrations of genistein activate STAT3 in prostate cancer cells.
The mechanisms by which STAT3 is activated by physiological concentrations of genistein is presently unknown. Several studies have confirmed that a variety of growth factors and cytokines, via the Jak and PI3K pathways, can activate STAT3 by phosphorylating tyrosine and serine residues ( 40 ). The hyperphosphorylation of STAT3 raises the possibility that physiologically achievable concentrations of genistein can alter the levels of some growth factors and/or cytokines and dysregulate ligand–receptor interactions and/or protein kinase activities to activate STAT3. As evidence that genistein affects kinases upstream of STAT3, we observed an increase in the expression of phosphorylated Akt (S473) with 1 μM of genistein treatment for 2 days (data not shown) and a decrease in the level of phosphorylated Akt (S473) with 10–100 μM of genistein treatment for 3 days in DU-145 cells ( 11 ). These results suggest that genistein inhibits kinases at pharmacological concentrations and activates kinases at physiological concentrations, which may explain the biphasic effect of genistein. Nonetheless, further experiments are necessary to fully validate the activation of STAT3 by Akt and to determine if there are other mechanisms for STAT3 activation by physiologically achievable concentrations of genistein.
To confirm the regulation of telomerase activity by STAT3, we examined the role of STAT3 on hTERT mRNA expression and transcriptional activity by over-expressing DN-STAT3. Our results are in agreement with Konnikova et al. ( 24 ) that the inhibition of STAT3 in prostate cancer cells decreases telomerase activity. Most interestingly, we observed that 1 μM of genistein increased STAT3 binding to the hTERT promoter and the inhibition of STAT3 repressed the genistein-induced increase in hTERT transcriptional activity. These results show for the first time that physiologically achievable concentrations of genistein enhanced the binding of activated STAT3 to the hTERT promoter and that STAT3 is necessary for the enhancement of hTERT transcriptional activity by genistein. Furthermore, it is possible that in addition to the direct regulation of the hTERT promoter, STAT3 may indirectly modulate telomerase activity via c-Myc as the regulation of telomerase activity by c-Myc has already been demonstrated ( 11 ).
In addition to increased hTERT transcriptional activity, the increased activation of STAT3 by genistein could modulate prostate cancer cell growth since STAT3 can regulate growth-promoting genes (cyclin D1 and c-Myc), anti-apoptotic genes (survivin, Bcl-2, Bcl-xL, Mcl-1 and Pim-1), an angiogenic gene (VEGF) and a gene associated with tumor invasion (LIV-1) ( 25 , 40 , 41 ). In this investigation, we observed that the protein levels of cyclin D1 and c-Myc were increased upon genistein treatment in DU-145 cells. These proteins can enhance the cell cycle, cell proliferation and hTERT transcriptional activity. Accordingly, we observed enhanced telomerase activity, cancer cell proliferation and tumor growth with physiological concentrations of genistein. Moreover, we recently reported that hTERT modulates the expression of cyclin D1 ( 42 ). Therefore, the simultaneous increase in cyclin D1 by genistein-induced activation of STAT3 directly (genistein → ↑STAT3 → ↑cyclin D1) and by hTERT indirectly (genistein → ↑STAT3 → ↑hTERT → ↑cyclin D1) could additively accelerate cancer growth.
TRAMP mice develop PIN by 12 weeks of age ( 36 ). Therefore, genistein treatment started when the TRAMP mice already had PIN lesions. The relevance of the timing of this treatment stems from the fact that at 12 weeks of age, the DLPs of TRAMP mice is in a highly proliferative stage that precedes the formation of a palpable tumor. This condition would be very hard to diagnose in human counterparts. Since the discovery of the promising chemopreventive aspects of genistein consumption, commercial soy supplements became readily available. However, emerging and alarming data suggest a cancer-promoting effect of genistein on other hormone-dependent cancers, such as breast cancer. This effect was seen in breast cancer cell lines as well as in animal models ( 43 , 44 ). Therefore, we were interested in determining the effect of genistein consumption on prostatic growth in the very early stages of malignancy, before the onset of palpable tumors; and we observed a significant increase in tumor growth with genistein consumption.
In conclusion, this study shows for the first time that physiologically achievable concentrations of genistein enhance growth in telomerase-positive human prostate cancer cells and a mouse model of prostate cancer but not in telomerase-negative normal human PrECs. Genistein enhances telomerase activity and hTERT transcriptional activity by activating STAT3 and increasing STAT3 binding to the hTERT promoter. These results are particularly alarming because there is a growing trend of consuming unknown amounts of phytoestrogen-containing dietary products and/or supplements for the prevention and/or treatment of cancer. This study raises a serious concern that instead of beneficial effects, certain physiologically achievable concentrations of genistein could have detrimental effects to individuals with prostate cancer.
Supplementary material
Supplementary Figures 1–4 can be found at http://carcin.oxfordjournals.org/ .
Funding
National Institutes of Health (R01 DK060875) to P.P.B.
Abbreviations
- DLPs
dorsolateral prostates
- TERT
telomerase reverse transcriptase
- mRNA
messenger RNA
- PCR
polymerase chain rection
- PrECs
prostate epithelial cells
- PIN
prostatic intraepithelial neoplasia
- RT
reverse transcriptase
- STAT
signal transducers and activators of transcription
- siRNA
small interfering RNA
- TEP1
telomerase-associated protein 1
- TERC
telomerase RNA
- TRAP
telomeric repeat amplification protocol
- TRAMP
transgenic adenocarcinoma mouse prostate
We would like to thank Dr Kimitoshi Kohno (Department of Molecular Biology, University of Occupational and Environmental Health, School of Medicine, Fukuoka, Japan) for providing the STAT3 promoter-luciferase construct, Dr Yoshiyasu Aoki (Experimental Transplantation and Immunology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD) for the DN-STAT3 vector, Dr James Darnell (Laboratory of Molecular Cell Biology, The Rockefeller University, New York, NY) for the CA-STAT3 and WT-STAT3 constructs and Dr Satoru Kyo (Department of Obstetrics and Gynecology, Kanazawa University, School of Medicine, Ishikawa, Japan) for the full-length hTERT-promoter luciferase plasmid.
Conflict of Interest Statement : None declared.
References
Author notes
These authors contributed equally to this work.