-
PDF
- Split View
-
Views
-
Cite
Cite
Stephen P. Faux, Catherine E. Houghton, Andrew Hubbard, Graham Patrick, Increased expression of epidermal growth factor receptor in rat pleural mesothelial cells correlates with carcinogenicity of mineral fibres, Carcinogenesis, Volume 21, Issue 12, 1 December 2000, Pages 2275–2280, https://doi.org/10.1093/carcin/21.12.2275
Close - Share Icon Share
Asbestos fibres have been shown to stimulate the mitogen-activated protein kinase signalling cascade in rat pleural mesothelial (RPM) cells after autophosphorylation of the epidermal growth factor receptor (EGFR). We examined if mineral fibres with known carcinogenicity can be discriminated from materials with less or no carcinogenicity by their ability to up-regulate expression of EGFR protein in RPM cells in vitro. Crocidolite and erionite, two fibrous preparations with marked potential to induce mesothelioma, were associated with increases in EGFR protein expression over sham controls, whereas chrysotile asbestos and milled (non-fibrous) crocidolite did not. Intense patterns of EGFR protein expression were linked to RPM cells phagocytosing long fibres. To determine the role of EGFR expression in these cells, we assessed cell proliferation using an antibody against proliferating cell nuclear antigen (PCNA) in combination with an antibody against EGFR. In these co-localization studies, cells showed intense staining for EGFR protein 24 h before being PCNA positive at 48 h. These results suggest that carcinogenic fibres induce EGFR and initiate cell signalling cascades in mesothelial cells, leading to cell proliferation and carcinogenesis.
Introduction
Occupational exposure to asbestos, a family of fibrous crystalline silicates, is the causative agent associated with the development of diffuse malignant mesothelioma, a fatal tumour arising from the mesothelial lining in the pleura and peritoneal cavities (1). Although mechanisms of fibre carcinogenicity are not well understood, several properties of fibres, such as their physical dimensions (length and diameter), biopersistence and surface reactivity, are thought to play an important role, contributing to chronic inflammation and unregulated proliferation of the target cells of the pleura (2).
Previous in vivo studies by Stanton et al. (3) have shown a relationship between fibre length and pathogenicity. These studies, with intrapleurally implanted fibres, showed that fibres >8 μm in length and <0.2 μm in diameter had greater ability to cause mesothelioma. In rodent inhalation and intraperitoneal instillation studies using two amosite asbestos samples, differing only in length, Davis et al. (4) demonstrated that the short and long fibres had dramatically differing pathogenic potential. In these studies the long fibres produced fibrosis, lung tumours and mesotheliomas, whereas the short fibres lacked pathogenic activity. Many fibres in the long-fibre amosite preparations in studies by Davis et al. (4) were in the `Stanton range', whilst there were no `Stanton' fibres in the short-fibre preparation. Complementary studies by Donaldson et al. (5) have shown that the long-fibre sample is many times more active than the short-fibre sample in producing inflammation, a characteristic response to carcinogenic mineral dusts.
Cell signalling events elicited by asbestos may lead to mesothelial cell proliferation, producing tumour initiation, promotion and progression. In this scheme of events, alterations in gene expression by asbestos initiated at the plasma membrane may facilitate chronic cell proliferation. Sustained cell proliferation appears to be a universal factor in human cancers (6) and in this process cancer appears to result from genetic errors induced or fixed during the process of cell division, as increased mitogenesis increases the risk of multiple genetic defects, including mutations, translocations and amplification of oncogenes. Recent studies in mesothelial cells have shown that asbestos fibres activate the `early response' proto-oncogenes c-fos and c-jun (7). Unlike the classical tumour promoter phorbol 12-myristate 13-acetate (TPA). TPA, which induces transient increases in c-fos and c-jun mRNA peaking at l h, crocidolite causes persistent induction of these proto-oncogenes and related transcription factors for ≥24 h and may serve as a chronic stimulation for cell proliferation after deposition of fibres in the lung or pleura. The induction of gene expression is dose-dependent and is not observed with polystyrene beads or riebeckite, which is chemically similar to crocidolite but non-fibrous, and suggests that fibre geometry is critical in proto-oncogene induction (8).
Interaction of fibres with cell surface receptors before the initiation of the signalling cascade in the regulation of immediate early genes may be important with asbestos. Mitogen-activated protein (MAP) kinases are members of a family of enzymes involved in regulating the response of eukaryotic cells to extracellular signals (9). Activation of MAP kinase is involved in the upstream events leading to c-fos and c-jun transcription because, after phosphorylation, MAP kinases, specifically extracellular signal-regulated kinases (ERKs), translocate to the nucleus phosphorylating substrates that are involved in the trans-activation of the proto-oncogenes (10). Recently, asbestos has been shown to cause stimulation of the ERK1 MAP kinase cascade after phosphorylation of the epidermal growth factor receptor (EGFR) (11). In these studies, crocidolite, but not its non-fibrous analogue riebeckite, induced MAP kinase activity that could be blocked by suramin (which has been shown to inhibit growth factor interactions) and by tyrphostin AG 1478, a specific inhibitor of the EGFR tyrosine kinase (12). It is possible that the EGFR plays a critical role in the carcinogenic process by asbestos. Studies have shown that the up-regulation of EGFR expression in NIH 3T3 cells leads to tumorigenicity (13) and increased expression of the EGFR has been found in several types of human malignancy, including mesothelioma (14). Whether asbestos fibres cause up-regulation of the EGFR in normal mesothelial cells is of considerable importance. The present studies have shown that both crocidolite asbestos and erionite fibres increase EGFR protein expression in normal rat pleural mesothelial cells and that this increased EGFR expression is associated with cell proliferation. In addition, milled crocidolite did not cause intense patterns of EGFR protein expression, indicating a specific association between receptor expression and the fibrous nature of crocidolite.
Materials and methods
Cell culture and exposure to test agents
Male Fischer 344 rats aged 12–20 weeks were killed by exsanguination under halothane anaesthesia and the thorax was opened. Rat pleural mesothelial (RPM) cells were isolated by gentle scraping of the parietal pleura as described by Jaurand et al. (15). Cells were propagated for four passages and maintained in Dulbecco's modified Eagle's medium (DMEM)/F-12, supplemented with penicillin (50 units/ml), streptomycin (100 μg/ml), containing 15% fetal bovine serum (FBS), hydrocortisone (100 ng/ml), insulin (2.5 μg/ml), transferrin (2.5 μg/ml) and selenium (2.5 ng/ml) as described by Heintz et al. (7). Cells were grown to confluency for nuclear protein preparations or 70% confluency on coverslips (no. 1, 19 mm diameter; Chance Proper Ltd, Warley, UK) for immunofluorescence studies; 24 h before exposure to mineral dusts or test agents, cells were switched to medium containing reduced serum (0.5% FBS). Mineral dusts were suspended in DMEM/F-12 at 1 mg/ml, sonicated for 4 min in a water bath, triturated eight times with a 22-gauge needle and then added to the medium to give final concentrations of 5.0 μg/cm2. Reference samples of NIEHS processed crocidolite (a kind gift from Brooke Mossman, Burlington, VT, USA), UICC chrysotile and milled crocidolite asbestos fibres were used. Erionite was obtained from Rome, OR, USA and a respirable sample prepared as described previously (16). Physical characteristics of the mineral fibres are given in Table I. Tumour necrosis factor α (TNFα) (Calbiochem, Nottingham, UK) and TPA (Sigma, Poole, UK) were added to the culture medium at final concentrations of 10 and 100 ng/ml, respectively.
lmmunofluorescence technique and confocal microscopy
RPM cells were cultured on coverslips as described above and switched to medium containing reduced serum (0.5% FBS) for 24 h before treatment with mineral fibres (5 μg/cm2) or TNFα [10 ng/ml in calcium- and magnesium-free phosphate-buffered saline (CMFPBS)/1% bovine serum albumin (BSA)] for 2, 8, 24, 48 or 72 h in the presence or absence of a 30 min pre-treatment with cycloheximide (10 μg/ml; Sigma). Cells were fixed in methanol for 20 min and permeabilized in 0.1% Triton X-100/4% FBS for 20 min. The cells were then blocked with CMFPBS containing 10% goat serum for 15 min to prevent non-specific binding of the antibodies. Cells were incubated with a rabbit polyclonal antibody against EGFR (2.5 μg/ml in CMFPBS/1% BSA; Santa Cruz Biotechnology, UK), a mouse monoclonal antibody against the active EGFR (2.5 μg/ml in CMFPBS/1% BSA; Chemicon International Ltd, Harrow, UK) and/or a proliferating cell nuclear antigen (PCNA) mouse monoclonal antibody (2.5 μg/ml in CMFPBS/1% BSA; Novocastra Laboratories Ltd, Newcastle upon Tyne, UK) for 12 h at room temperature. The negative control coverslips were incubated with CMFPBS/1% BSA in the absence of primary antibody. The cells were then labelled with a rhodamine-linked (TRITC) goat anti-rabbit immunoglobulin G (2.5 μg/ml in CMFPBS/1% BSA) and/or a fluorescein-isothiocyanate (FITC)-linked goat anti-mouse immunoglobulin G (100 μg/ml in CMFPBS/1% BSA) for 1 h at 37°C in 5% CO2. The coverslips were mounted on microscope slides using Fluoromount (BDH, Lutterworth, UK) and kept at 4°C until analysis by confocal microscopy.
A Leica True Confocal Scanner 4D was used to visualize and locate the EGFR in RPM cells. The microscope was used to scan simultaneously in two channels, at 488 and 568 nm, to excite fluorescein and rhodamine, respectively. Throughout the experiments, levels of laser emission were kept the same for all the samples and both photomultiplier tube settings were kept constant. The respective emissions were detected and cell preparations were optically sectioned. Optical data stacks were combined into a maximum projection and stored as true colour 24-bit digital images. The digital images were imported into Adobe Photoshop software and colour output was obtained using a Kodak dye sublimation printer.
Isolation of nuclear proteins and electrophoretic mobility shift assays of AP-1 DNA binding
RPM cells were treated with crocidolite asbestos (2.5 and 5 μg/cm2) for 8 h or with TPA (100 ng/ml) for 1 h, in the presence or absence of a 1 h pre-incubation with suramin (20 μM; Calbiochem) or tyrphostin AG 1478 (10 μM; Calbiochem), a selective inhibitor of EGFR, before isolation of nuclear protein extracts as described by Staal et al. (17). Briefly the cells were washed in buffer [10 mM HEPES/KOH (pH 7.5), 10 mM KCl, 2 mM MgCl2, 1 mM dithiothreitol (DTT), 0.1 mM EDTA, 0.4 mM phenylmethylsulfonyl fluoride (PMSF), 0.2 mM NaF, 0.2 mM sodium orthovanadate and 0.3 mg/ml leupeptin] at 4°C for 15 min and lysed in the same buffer containing 1% Triton X-100. Nuclei were pelleted by centrifugation at 14 000 r.p.m. for 30 s at 4°C and resuspended in high salt buffer [50 mM HEPES (pH 7.8), 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 0.4 mM PMSF, 0.2 mM NaF, 0.2 mM sodium orthovanadate and 10% glycerol]. Nuclear proteins were then extracted by centrifugation at 14 000 r.p.m. for 5 min at 4°C; the supernatant was designated as the nuclear protein extract and stored at –80°C. Electrophoretic mobility shift assays (EMSAs) were performed using procedures described previously (18). Briefly, 4 μg of a nuclear protein extract was incubated for 20 min at room temperature in DNA binding buffer containing 40 mmol/1 HEPES buffer, 4% Ficoll, 200 ng of poly(dI)˙(dC) per μl, 1 mmol/1 MgCl2, 0.1 mmol/1 DTT and 0.175 pmol of a 32P-end-labelled double-stranded oligonucleotide containing a consensus activator protein-1 (AP-1) site (Promega, Southampton, UK). Samples were loaded on to a 4% polyacrylamide gel and electrophoresed in 0.25× Tris–borate–EDTA buffer for 2 h at 120 V. Gels were dried for 1 h at 80°C and visualized by autoradiography by exposure to Kodak X-Omat film (Sigma). Subsequently, radioactivity in retarded binding complexes was quantified on a Phosphoimager (Molecular Dynamics, UK). The specificity of AP-1 DNA-binding patterns was characterized using a 10-fold excess of unlabelled AP-1 oligonucleotide that competed away all binding complexes, whereas excess of nuclear factor-κB (NF-κB) oligonucleotide did not modify gel-shift complexes, demonstrating the specificity of AP-1 gel-shift patterns.
Results
Immunofluorescence detection of EGFR protein by confocal laser-scanning microscopy
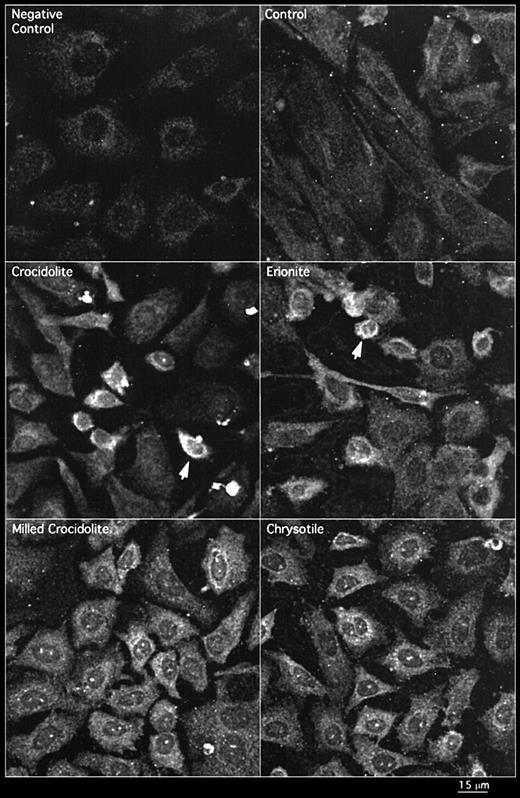
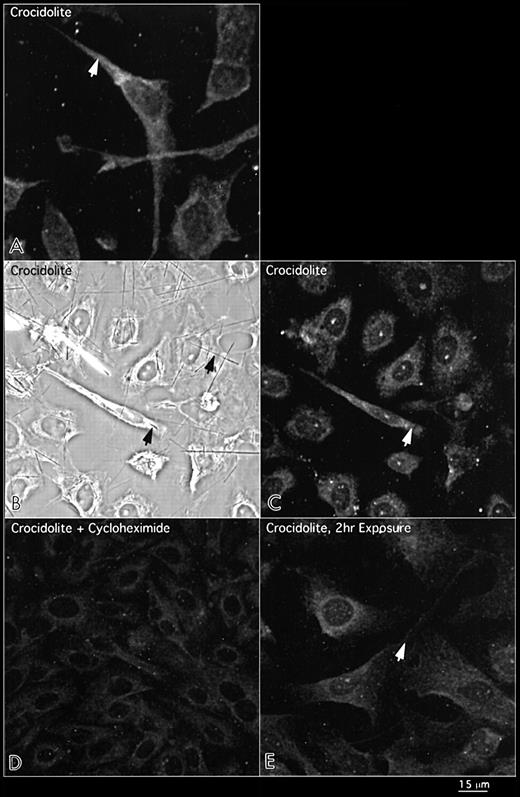
To determine if mineral dusts increase EGFR protein expression in RPM cells, we have used a combination of fluorescence and reflectance confocal laser-scanning microscopy (CLSM) to examine the immunolocalization of EGFR in these cells. Crocidolite and erionite, two fibrous preparations known to induce mesothelioma, increased EGFR protein expression in RPM cells over the sham controls, inducing patterns of intense expression. In contrast, milled crocidolite (non-fibrous) and chrysotile asbestos, two preparations with no or much less ability to induce mesothelioma, did not increase the intensity of immunostaining for EGFR protein over the sham control (Figure 1; Table II). By comparing the confocal fluorescence image, indicating EGFR immunoreactivity (Figure 2C), with a CLSM image demonstrating fibre morphology (Figure 2B), we have shown that cells attempting to phagocytose long crocidolite fibres showed increased staining for EGFR and acquired a fusiform shape, whereas cells in contact with a number of shorter fibres showed negligible immunostaining for EGFR and remained polygonal in morphology.
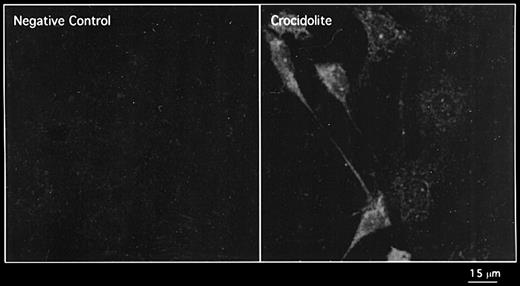
To investigate whether crocidolite asbestos increases the amount of newly synthesized EGFR protein rather than redistributing protein within RPM cells, we incubated cells with crocidolite for 8 h after pre-treatment for 30 min with cycloheximide, an inhibitor of protein synthesis, and with crocidolite for 2 h. In these experiments, cycloheximide abrogated crocidolite-mediated EGFR protein induction and at 2 h, immunostaining for EGFR protein was not different from that in the sham controls (Figure 2D and E, respectively). Up-regulation of EGFR protein expression was only observed after 8 h fibre exposure and continued for ≥72 h. To determine if crocidolite up-regulates phosphorylated EGFR (pEGFR), we incubated with an antibody against this active form of the receptor. In these studies at 24 h, expression of pEGFR protein was greater in crocidolite-treated cells than in sham controls (Figure 3).
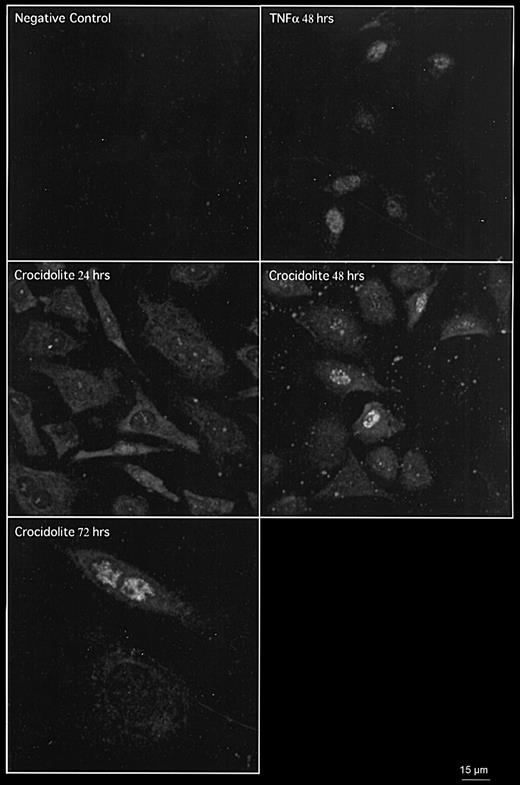
To determine if cell proliferation is the phenotypic outcome of asbestos-mediated EGFR protein expression, as EGF is a potent mitogen in mesothelial cells, we assessed the immunoreactivity of antibodies against EGFR and PCNA, a marker of cell proliferation, in mesothelial cells exposed to crocidolite, by dual fluorescence labelling with secondary antibodies linked to rhodamine (red) and fluorescein (green), respectively. Crocidolite increased EGFR protein expression at 24 h and both EGFR and nuclear PCNA at 48 and 72 h exposure, with TNFα as a positive control for PCNA at both time-points (Figure 4; data for 72 h not shown). No increase in PCNA expression was observed at 8 h (data not shown) and 24 h.
EMSA analyses of AP-1–DNA binding in mesothelial cells
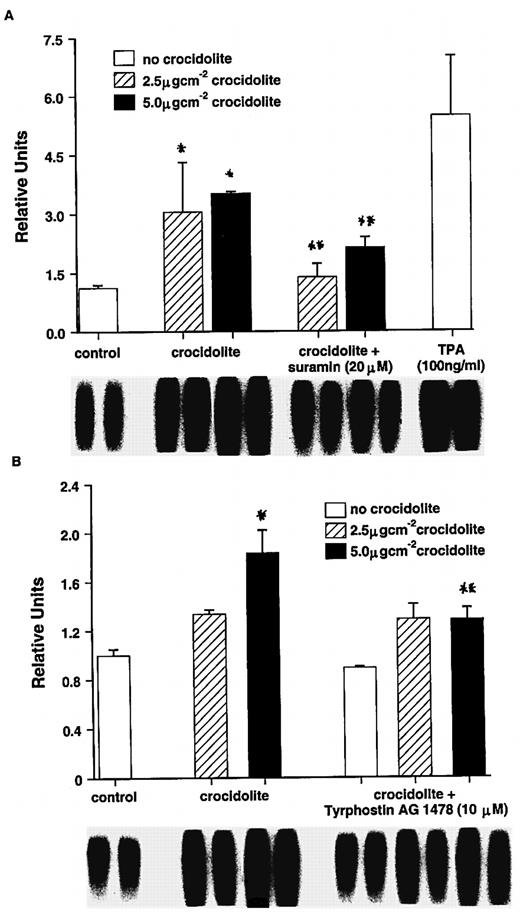
To determine if crocidolite asbestos induces AP-1–DNA binding in RPM cells via a mechanism dependent on expression of EGFR protein, we incubated RPM cells in the presence of suramin or tyrphostin AG 1478, inhibitors of membrane receptor binding and EGFR, respectively. In RPM cells treated in the presence of suramin, crocidolite-mediated increases in AP-1–DNA binding activity were significantly reduced (P < 0.05, Figure 5A). A similar effect was observed when suramin was replaced by tyrphostin AG 1478 in the incubation (P < 0.05; Figure 5B).
Discussion
The pathogenicity of mineral fibres has been attributed to both chemical and physical properties of these fibres. The high iron content of the asbestiform mineral fibres may drive redox reactions on the fibre surface, producing reactive oxygen species (ROS), which are both mutagenic and mitogenic (19–21). Oxidant-dependent and -independent mechanisms may explain why asbestos fibres over a certain length (8 μm) are more potent in inducing mesothelioma than shorter fibres (4). Larger asbestos fibres are able to induce greater release of ROS from alveolar and peritoneal macrophages than shorter fibres, which is thought to be due to incomplete phagocytosis (22). Asbestos fibres, but not their non-fibrous analogues, can directly trigger cell signalling pathways in mesothelial cells that are initiated at the cell surface (11,23). These recent studies show that activation of the MAP kinase cascade in mesothelial cells (11) occurs after a number of phosphorylation events, including autophosphorylation of the EGFR. Subsequently, there is activation of transcription factors such as NF-κB (24) and AP-1 (7), which are associated with the trans-activation of intermediate early response genes important in cell proliferation and carcinogenesis.
In the present studies we have shown for the first time in normal RPM cells that crocidolite asbestos and erionite, two chemically different fibres both known to induce mesothelioma in rats and humans, increase the expression of a growth factor receptor protein, EGFR, whereas non-fibrous milled crocidolite, which does not cause mesothelioma, did not increase the expression of EGFR protein in RPM cells. We also compared the effect of chrysotile fibres and found that they did not cause intense immunostaining for EGFR protein expression. As recent reviews have indicated, the ability of chrysotile to induce mesothelioma in humans is still vigorously debated (25,26). It is agreed, however, that amphiboles such as crocidolite are much more potent than chrysotile. This difference is not always apparent in animal inhalation studies, but there are differences according to the source of the chrysotile, in particular whether it is contaminated with the amphibole tremolite (27). Erionite has consistently proved more potent than all other fibres in this type of study (28).
Even more diverse findings have been obtained after fibres have been injected intrapleurally, when they come into immediate contact with mesothelial cells. In one sense, of course, this mode of administration is more relevant to the present in vitro study, where pleural mesothelial cells are exposed directly to the fibres. Chrysotile has frequently produced mesotheliomas in these circumstances, but where UICC chrysotile B has been compared with UICC crocidolite, the latter is more potent at inducing mesothelioma (29). Other forms of chrysotile have sometimes been as effective as crocidolite (30,31). Similarly, after intraperitoneal injection, UICC chrysotile B was less effective than crocidolite, though this was without statistical significance (32), and other forms of chrysotile could be as effective or more so (33). Again erionite is always the most effective fibre (28,34). From this we conclude that the difference in EGFR expression in vitro between UICC crocidolite and UICC chrysotile B is consistent with their relative potency in inducing mesothelioma in vivo.
Evidence that new protein is expressed after asbestos exposure is provided by experiments using cycloheximide, an inhibitor of protein synthesis (35). Because crocidolite-mediated receptor expression is abrogated in the presence of cycloheximide, we conclude that crocidolite asbestos fibres cause increased expression of EGFR protein, and not just altered distribution of the growth factor receptor protein.
Our observations in normal RPM cells differ in the time-frame of induction of EGFR protein from previous observations in MET 5A cells, a human mesothelial cell line (36). In the present studies, up-regulation of EGFR protein was only observed after 8 h exposure, compared with 2 h in MET 5A cells. A factor that may be important in accounting for this difference is that MET 5A cells are transformed with the simian virus 40 (SV40) T antigen, which is known to alter in vitro cellular function and cause transformation (37). The mechanisms by which this viral antigen enhances the transformation of resting cells may be related to its ability to stimulate or repress the transcription of certain genes. Thus, a wide variety of proto-oncogenes encoding growth factors and growth factor receptors could be affected by SV40 in combination with asbestos and the effect of SV40 on EGFR expression by asbestos cannot be ruled out in the investigations using the MET 5A cell line (36).
It has been demonstrated in a number of cell types that the addition of epidermal growth factor (EGF) causes autophosphorylation of the EGFR that in turn activates the MAP kinase cascade linked to trans-activation of proto-oncogenes important in mitogenesis (38). In the present studies we have shown that crocidolite asbestos up-regulates the phosphorylated form of the EGFR by immunofluorescence using a monoclonal antibody directed against the active form of this growth factor receptor. Mesothelial cell fibre interactions lead to a number of phosphorylation events associated with the EGFR signal transduction pathway, involving MAP kinase. The functional implication of EGFR protein expression has not previously been determined with crocidolite asbestos. In contrast to a number of other growth factors, addition of EGF to mesothelial cells induces cell proliferation (39). In the present work, we have shown by co-immunofluorescence that expression of EGFR protein in mesothelial cells precedes the expression of PCNA, a marker of cell proliferation, following exposure to crocidolite asbestos.
Downstream events following the initiation of cellular signalling events at the plasma membrane by asbestos involve the activation of transcription factors, such as AP-1, that are important in cell proliferation (40). Using both suramin, a compound that uncouples G-proteins from receptors and prevents receptor function (41) and tyrphostin AG 1478, a potent and selective inhibitor of EGFR itself (42), we have shown that the activation of growth factor receptors, notably EGFR, by asbestos is crucial to the initiation of signalling events leading to AP-1 induction in mesothelial cells by asbestos.
In summary, we have shown that fibres with the greatest potential to induce mesothelioma show the most marked up-regulation and activation of EGFR in RPM cells. This supports the view that chronic expression of EGFR protein and subsequent proliferation of mesothelial cells are involved in the chain of events leading from asbestos exposure to neoplastic progression.
Physical characteristics of the mineral fibres
| Mineral fibre preparation . | Mean diameter (μm) . | Mean length (μm) . |
|---|---|---|
| aResults obtained from scanning electron microscopy. | ||
| bNot determined, as no fibres were observed by scanning electron microscopy. | ||
| Crocidolitea | 0.27 | 11.4 |
| Milled crocidoliteb | ND | ND |
| Erionitea | 0.8 | 6.0 |
| Chrysotilea | 0.04 | 1.1 |
| Mineral fibre preparation . | Mean diameter (μm) . | Mean length (μm) . |
|---|---|---|
| aResults obtained from scanning electron microscopy. | ||
| bNot determined, as no fibres were observed by scanning electron microscopy. | ||
| Crocidolitea | 0.27 | 11.4 |
| Milled crocidoliteb | ND | ND |
| Erionitea | 0.8 | 6.0 |
| Chrysotilea | 0.04 | 1.1 |
Physical characteristics of the mineral fibres
| Mineral fibre preparation . | Mean diameter (μm) . | Mean length (μm) . |
|---|---|---|
| aResults obtained from scanning electron microscopy. | ||
| bNot determined, as no fibres were observed by scanning electron microscopy. | ||
| Crocidolitea | 0.27 | 11.4 |
| Milled crocidoliteb | ND | ND |
| Erionitea | 0.8 | 6.0 |
| Chrysotilea | 0.04 | 1.1 |
| Mineral fibre preparation . | Mean diameter (μm) . | Mean length (μm) . |
|---|---|---|
| aResults obtained from scanning electron microscopy. | ||
| bNot determined, as no fibres were observed by scanning electron microscopy. | ||
| Crocidolitea | 0.27 | 11.4 |
| Milled crocidoliteb | ND | ND |
| Erionitea | 0.8 | 6.0 |
| Chrysotilea | 0.04 | 1.1 |
Numbers of RPM cells showing mild or intense EGFR protein expression as detected by immunofluorescence with confocal laser-scanning microscopy
| Mineral fibre preparation . | % of cells staining for EGFR protein . | |
|---|---|---|
| . | mild staining . | intense staining . |
| The results are the mean ± SD of 10 random fields (20–30 cells counted/field/coverslip in duplicate). | ||
| Control | 10 ± 4 | 0 |
| Crocidolite | 11.54 ± 6.31 | 45.46 ± 4.54 |
| Milled crocidolite | 11.41 ± 3.17 | 0 |
| Erionite | 10 ± 7 | 40 ± 7.62 |
| Chrysotile | 16.66 ± 8.66 | 0 |
| Mineral fibre preparation . | % of cells staining for EGFR protein . | |
|---|---|---|
| . | mild staining . | intense staining . |
| The results are the mean ± SD of 10 random fields (20–30 cells counted/field/coverslip in duplicate). | ||
| Control | 10 ± 4 | 0 |
| Crocidolite | 11.54 ± 6.31 | 45.46 ± 4.54 |
| Milled crocidolite | 11.41 ± 3.17 | 0 |
| Erionite | 10 ± 7 | 40 ± 7.62 |
| Chrysotile | 16.66 ± 8.66 | 0 |
Numbers of RPM cells showing mild or intense EGFR protein expression as detected by immunofluorescence with confocal laser-scanning microscopy
| Mineral fibre preparation . | % of cells staining for EGFR protein . | |
|---|---|---|
| . | mild staining . | intense staining . |
| The results are the mean ± SD of 10 random fields (20–30 cells counted/field/coverslip in duplicate). | ||
| Control | 10 ± 4 | 0 |
| Crocidolite | 11.54 ± 6.31 | 45.46 ± 4.54 |
| Milled crocidolite | 11.41 ± 3.17 | 0 |
| Erionite | 10 ± 7 | 40 ± 7.62 |
| Chrysotile | 16.66 ± 8.66 | 0 |
| Mineral fibre preparation . | % of cells staining for EGFR protein . | |
|---|---|---|
| . | mild staining . | intense staining . |
| The results are the mean ± SD of 10 random fields (20–30 cells counted/field/coverslip in duplicate). | ||
| Control | 10 ± 4 | 0 |
| Crocidolite | 11.54 ± 6.31 | 45.46 ± 4.54 |
| Milled crocidolite | 11.41 ± 3.17 | 0 |
| Erionite | 10 ± 7 | 40 ± 7.62 |
| Chrysotile | 16.66 ± 8.66 | 0 |
Expression of EGFR protein in RPM cells treated with crocidolite, erionite, milled crocidolite and chrysotile as shown by immunofluorescence detected by CLSM. The arrows indicate cells showing increases in immunoreactive protein.
Distribution of the external domain of the EGFR protein in RPM cells exposed to crocidolite asbestos for 8 h as indicated by the arrows in the top panels (A). The middle panels show both phase microscopy (B) and immunofluorescence (C), illustrating the fusiform shape of cells encompassing long crocidolite fibres and expressing intense patterns of EGFR protein. Cells exposed to a number of shorter fibres remain polygonal with no increase in EGFR protein expression. The bottom panels show inhibition of crocidolite-mediated EGFR protein with cycloheximde at 8 h (D) and no increase in EGFR protein after 2 h exposure to crocidolite (E).
Expression of the phosphorylated form of the EGFR protein in RPM treated with crocidolite for 8 h as shown by immunofluorescence detected by CLSM.
Crocidolite induces EGFR expression at 24, 48 and 72 h and PCNA expression at 48 and 72 h in RPM cells as shown by co-immunofluorescence detected by CLSM. TNFα was used as a positive control for PCNA expression in RPM cells at 48 h.
Effect of suramin (A) and the EGFR inhibitor tyrphostin AG 1478 (B) on crocidolite-mediated AP-1–DNA binding activity in RPM cells: *Significantly different from untreated controls (P < 0.05): **Significantly different from crocidolite alone (P < 0.05).
To whom correspondence should be addressed
The authors thank Professor Andy Gescher and Drs Maggie Manson and Tim Gant (MRC Toxicology Unit, Leicester, UK) for critical reading of the manuscript.
References
Wagner,J.C., Sleggs,C.A. and Marchant,P. (
Mossman,B.T., Bignon,J., Corn,M., Seaton,A. and Gee,J.B.L. (
Stanton,M.F., Layard,M. and Tegeris,A. (
Davis,J.M.G., Bolton,R.E., Donaldson,K., Jones,A.D. and Smith,T. (
Donaldson,K., Brown,G.M., Brown,D.M., Bolton,R.E. and Davis,J.M.G. (
Preston-Martin,S., Pike,M.C., Ross,R.K., Jones,P.A. and Henderson,B.E. (
Heintz,N.H., Janssen,Y.M.W. and Mossman,B.T. (
Janssen,Y.M.W., Heintz,N.H., Marsh,J.P., Borm,P.J.A. and Mossman,B.T. (
Boulton,T.G., Yancopoulos,G.D., Gregory,J.S., Slaughter,C., Moomaw,C., Hsu,J. and Cobb,M.H. (
Zinck,R., Hipskind,R.A., Pingoud,V. and Nordheim,A. (
Zanella,C.L., Posada,J., Tritton,T.R. and Mossman,B.T. (
Levitzli,A. and Gazit,A. (
Hudziak,R.M., Schlessinger,J. and Ullrich,A. (
Dazzi,H., Hasleton,P.S., Thatcher,N., Wilkes,S., Swindell,R. and Chatterjee,A.K. (
Jaurand,M.C., Bastie-Sigeac,J., Bignon,J. and Stoebner,P. (
Poole,A., Brown,R.C., Turver,C.J., Skidmore,J.W. and Griffths,D.M. (
Staal,F.J.T., Roederer,M., Herzenberg,L.A. and Herzenberg,L.A. (
Faux,S.P. and Howden,P.J. (
Faux,S.P., Howden,P.J. and Levy,L.S. (
Howden,P.J. and Faux,S.P. (
Donaldson,K., Slight,J. and Bolton,R. (
Jimenez,L.A., Zanella,C.L., Fung,H., Janssen,Y.M.W.,Vacek, P., Charland,C., Goldberg,J. and Mossman,B.T. (
Janssen,Y.M.W., Driscoll,K.E., Howard,B., Quinlan,T.R., Treadwell,M., Barchowsky,A. and Mossman,B.T. (
Harrison,P.T.C., Levy,L.S., Patrick,G., Pigott,G.H. and Smith,L.L. (
Landrigan,P.J., Nicholson,W.J., Suzuki,Y. and Ladou,J. (
Lippmann,M. (
Wagner,J.C., Skidmore,J.W., Hill,R.J. and Griffiths,D.M. (
Wagner,J.C., Berry,G. and Timbrell,V. (
Wagner,J.C. and Berry,G. (
Stanton,M.F. and Wrench,C. (
Pott,F. (
Davis,J.M.G., Bolton,R.E., Miller,B.G. and Niven,K. (
Carthew,P., Hill,R.J., Edwards,R.E. and Lee,P.N. (
Obrig,T.G., Culp,W.J., McKeehan,W.L. and Hardesty,B. (
Pache,J.-C., Janssen,Y.M.W., Walsh,E.S., Quinlan,T.R., Zanella,C.L., Low,R.B., Taatjes,D.J. and Mossman,B.T. (
Sontag,E., Sontag,J.-M. and Garcia,A. (
Cobb,M.H. and Goldsmith,E.J. (
Gabrielson,E.W., Gerwin,B.I., Harris,C.C., Roberts,A.B., Sporn,M.B. and Lechner,J.R. (
Angel,P. and Karin,M. (
Kopp,R. and Pfeiffer,A. (