-
PDF
- Split View
-
Views
-
Cite
Cite
Chun-Nan Yeh, Anirban Maitra, Kam-Fai Lee, Yi-Yin Jan, Miin-Fu Chen, Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: an animal model recapitulating the multi-stage progression of human cholangiocarcinoma, Carcinogenesis, Volume 25, Issue 4, April 2004, Pages 631–636, https://doi.org/10.1093/carcin/bgh037
Close - Share Icon Share
Abstract
Cholangiocarcinoma (CCA) is a lethal disease, afflicting many thousands the world over. Human CCA develops through a multi-step progression model, preceded by the onset of dysplasia in the cholangiolar ductal epithelium. An animal model of multi-step carcinogenesis in the biliary tree will enable the study of genetic changes in human CCA, and provide an avenue for chemoprevention strategies. We describe an oral thioacetamide (TAA)-induced model of rat CCA that recapitulates the histologic progression of human CCA. Male Sprague–Dawley (SD) rats ( n = 170), weighing 350 ± 20 g, were used in this study. Drinking water with TAA 300 mg/l was administered orally, and the liver was harvested and examined histologically at weekly intervals, beginning at 5 weeks after initiation of TAA. Harvested tissues were formalin-fixed and paraffin embedded for morphologic and immunohistochemical studies. Multifocal bile ductular proliferation with intestinal metaplasia (presence of goblet cells) and increasing histologic atypia (biliary dysplasia) was observed by the 9th week of TAA administration. Biliary cytokeratin (CK19)-expressing invasive intestinal-type CCA with stromal desmoplasia was evident at the 16th week, and by the 22nd week, the yield rate for CCAs had increased to 100%. Invasive CCAs preceded the development of hepatic cirrhosis by at least 4 weeks; the earliest incidence of hepatic fibrosis was observed beginning at 20 weeks post-TAA administration. The progression from normal cholangioles to biliary dysplasia to invasive CCA was accompanied by up-regulation of the proto-oncogenes c-met and c-erbB-2, tyrosine kinase receptors over-expressed in human CCAs. The study was terminated at 6 months, at which time no systemic metastases or deaths were observed. Oral administration of TAA in drinking water to male SD rats provides a reproducible animal model for development of CCA with a high yield rate. In particular, the presence of biliary dysplasia beginning at the 9th week, which progresses to invasive CCA, mimics the multi-step model of human CCA. The TAA rat model may serve as a powerful pre-clinical platform for therapeutic and chemoprevention strategies for human CCA.
Introduction
Cholangiocarcinoma (CCA) is a lethal disease, afflicting approximately 3000 individuals in the US and many thousands more the world over ( 1 ). The mortality from intrahepatic CCAs is very high, with the 5-year survival rates being <15–20% in most series ( 2 ). There are numerous risk factors for CCA including primary sclerosing cholangitis, parasitic infections, choledochal cysts, hepatolithiasis and carcinogen exposure ( 2 , 3 ). Increasing evidence suggests that human CCA proceeds through a multi-step process, and that invasive CCA is preceded by dysplasia in the biliary epithelium ( 4 – 6 ). The molecular and genetic alterations involved in the CCA tumorigenesis have not been well investigated. However, recent studies suggest that alterations in the tyrosine kinase receptors c-Erb-2/c-Neu and c-Met, together with possible aberrant autocrine expression of hepatocyte growth factor/scatter factor, the ligand for c-Met, may be playing important roles associated with the pathogenesis of human CCA ( 7 – 9 ).
The development of a reproducible animal model for CCA, particularly one that recapitulates the dysplasia-carcinoma sequence of human CCA, would not only enhance our understanding of genetic changes underlying cholangiocellular neoplasia, but also facilitate the development of pre-clinical chemoprevention and therapeutic trials. We have developed a rat model of CCA using orally administered thioacetamide (TAA). Unlike previously described models of TAA carcinogenesis that have primarily focused on hepatocellular neoplasms ( 10 – 12 ), we demonstrate that oral administration of TAA results in early and profound dysplastic changes in the biliary epithelium, which subsequently progresses to biliary cytokeratin (CK19)-expressing invasive CCA. In addition to recapitulating the dysplasia-carcinoma sequence, this model is also associated with up-regulation of the proto-oncogenes c-met and c-erbB2 in the cholangiolar epithelium, mimicking one of the most common genetic changes seen in human CCAs ( 7 – 9 ).
Materials and methods
Experimental protocol
The experimental animal ethics committee of Chang Gung Memorial Hospital approved all animal protocols in this study. Furthermore, the investigation conformed to the US National Institute of Health (NIH) guidelines for the care and use of laboratory animals (Publication No. 85-23, revised 1996). 170 adult male Sprague–Dawley (SD) rats (330–370 g) were used in these experiments. The animals were divided into two groups, including a control group ( n = 10) and an experiment group ( n = 160). The rats were housed in an animal room with a 12:12-h light–dark cycle (light from 08:00 to 20:00) at an ambient temperature of 22 ± 1°C, with food and water available ad libitum . The experiment group rats were administered 300 mg TAA/l in their drinking water every day up to the time they were killed (as described below).
In the experiment group, eight animals were harvested weekly during the study to examine the effect of TAA and establish the natural history of the animal model. During the experiment, the animals were weighed weekly to calculate their body weight gain. Furthermore, blood samples were drawn from the inferior vena cava, and biochemistry tests: total protein, albumin, aspartate aminotransferase (AST), alkaline phosphatase (ALK), bilirubin and prothrombin time (PT) were determined using an automated technique.
Harvesting procedure and histopathological evaluation of the liver
The animals were anesthetized using ketamine (50 mg/kg, intraperitonerally, supplemented with a 20 mg/kg/h i.v. infusion). The animals were allowed to spontaneously breathe room air, and their rectal temperature was maintained at 37°C with a heating pad. Following a midline laparotomy, all lobes of the liver were explored and thoroughly examined to clarify the yield rate of CCA at every time point. The inferior vena cava was used to take a blood sample to measure the liver enzyme and prothrombin time. The liver was perfused with a freshly prepared phosphate buffered saline (pH 7.2) containing 4% paraformaldehyde under a constant pressure of 10 cm H 2 O for 5 min through the portal vein, cut into 3–5-mm sections, and stored in 70% alcohol after an additional fixation in 4% paraformaldehyde.
Immunohistochemical staining for c-met and c-erbB2
Staining with hematoxylin and eosin and immunohistochemical analyses were performed on 4-µm serial sections of paraffin-embedded, formalin-fixed tissue. Immunostaining was performed at room temperature and carried out on the Dako Autostainer (Dako-Carpinteria, TOWN/CITY, CA). Reagents were used as supplied in the EnvisionPlus Detection Kit, (Dako-Carpinteria). Dako Target Retrieval Solution, pH 6.0 was used. Sections were incubated with primary monoclonal antibody against c-Met (clone DO-7, Dako, TOWN/CITY, Denmark, dilution 1:100) and c-erb-B2 (clone sc-284, Santa Cruz Biotechnologies, Santa Cruz, CA, dilution 1:100). The slides were examined by two authors on the panel (C.-N.Y., A.M.) and labeling of cholangiolar epithelium was scored as negative (<5% staining); focal positive (5–25% staining) and diffuse (>25% staining), as described previously ( 9 ).
Statistics
All data are presented as means with standard deviations, and differences between the study and control groups are determined using an independent two-sample t test, and an ANOVA test when appropriate. All statistical analysis was performed using SPSS computer software (Chicago, IL), with a P value of <0.05 being considered statistically significant.
Results
Systemic effects of TAA administration
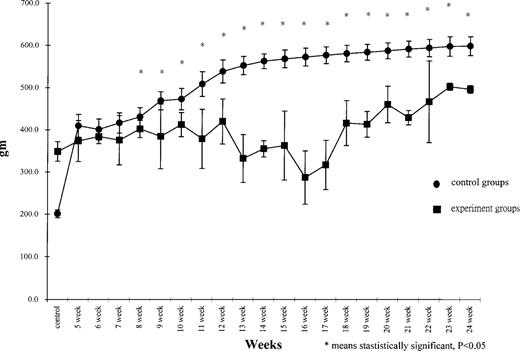
There were no instances of TAA-induced mortality during the 6-month study period. Significantly lower body weight gain was observed in the TAA fed rats compared with the control rats beginning at 8 weeks post-treatment ( Figure 1 ). Biochemistry test levels, including total protein, albumin, AST, ALK, bilirubin and PT were observed to be similar in both groups.
Body weight gain of rats at different time periods post-treatment.
TA administration induces early and profound dysplasia in the cholangiolar epithelium
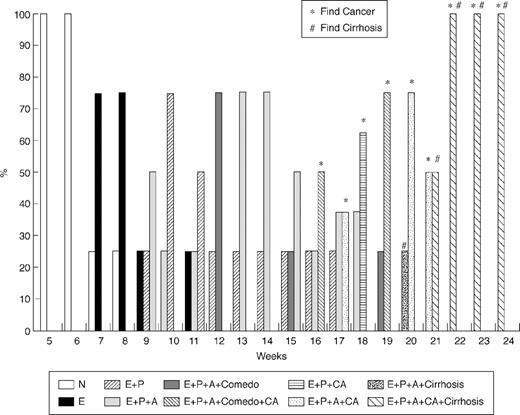
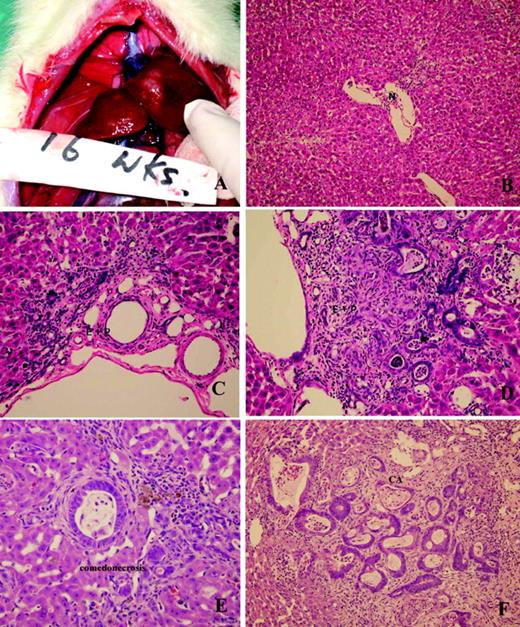
During the first 6 weeks following the administration of TAA, there was no evidence of either hepatocellular or cholangiocellular toxicity by gross and light microscopic examination. By the 9th week, however, multifocal bile ductular proliferation with demonstrable atypia (biliary dysplasia) was observed in 50% of the rats. The histologic features included proliferating ductules with abnormal luminal profiles, enlarged nuclei with hyperchromasia and pleomorphism, conspicuous nucleoli and loss of nuclear polarity. Biliary dysplasia was accompanied in many instances by metaplastic intestinal-type goblet cells. The latter has been described as a precursor lesion in the subset of ‘intestinal-type’ CCAs in humans ( 3 ), and is a frequently observed feature in other described models of rat CCA ( 13 ). Stromal desmoplasia was not present at this point in time, neither was hepatocellular toxicity seen on light microscopy. Progression of atypia without evidence of invasion was observed until the 16th week, at which time 50% of the animals developed multiple white, round and firm nodules over the hepatic surface, including the left, middle and right liver lobes. Histopathology revealed invasive intestinal-type CCA with intense stromal desmoplasia. The neoplastic glands exhibited all the histologic features described above, including the presence of goblet cells, but in addition, demonstrated prominent intra-luminal necrosis (‘comedonecrosis’), an indication of high cellular turnover. Intense expression of biliary cytokeratin, CK19, was seen in all neoplastic glands. Temporal heterogeneity in the biliary lesions was observed, with post-16th week harvested livers demonstrating both invasive CCA and non-invasive, but dysplastic, glands at any given time. From the 16th to 22nd week, the incidence of invasive CCAs increased progressively to 100% for livers harvested ( Figures 2 and 3 ).
Temporal progression of changes in the liver post TAA treatment. (N: normal; E: enlargement; P: proliferation; A: atypia; Comedo: comedonecrosis; CA: carcinoma).
Gross and microscopic features of multi-step progression model of rat CCA. ( A ) Gross tumor nodules (16 weeks). ( B ) Normal cholangiole (6 weeks) (×100, H&E stain). ( C ) Cholangiolar proliferation without atypia (8 weeks) (×200, HE stain). ( D ) Cholangiolar proliferation with atypia (10 weeks) (×200, H&E stain). ( E ) Atypical cholangiolar proliferation with comedonecrosis (15 weeks) (×200, HE stain). ( F ) Invasive intestinal-type CCA with intense stromal desmoplasia (16 weeks) (×200, H&E stain).
The first demonstrable hepatocellular pathology was observed at the 20th week post-initiation of TAA therapy. Increasing hepatic fibrosis, culminating in macronodular cirrhosis was seen, as a well-known toxicity of TAA ( 14 ). The study was terminated at 6 months, at which point no systemic metastases were observed from the CCA lesions.
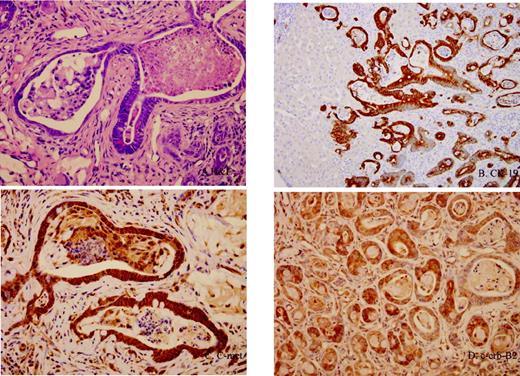
Immunohistochemical analysis for both c-Met and c-erb-B2 expression confirmed up-regulation of the proto-oncogene in epithelial cells of both early dysplastic glands (as early as the 9th week) as well as in the later invasive CCAs, compared with normal ductules. Intense, diffuse C-met and c-erb-B2 expression was observed in the dysplastic and neoplastic glands, while expression was minimal to absent in normal ductular epithelium ( Figure 4 ).
Immunohistochemical analyses of multi-step progression model of rat CCA. ( A ) Invasive intestinal-type CCA with intense stromal desmoplasia (×400, H&E stain). ( B ) Expression of biliary cytokeratin, CK19, in all neoplastic glands (×100). ( C ) c-met expression in cancerous biliary epithelium (×200). ( D ) c-erb-B2 expression in cancerous biliary epithelium (×200).
Discussion
Several environmental carcinogenesis models of CCA have been established in animals. For example, Syrian hamsters were treated with Clonorchis sinensis or Opisthorchis viverrini followed by dimethylnitrosamine (DMN) results in the development of CCA ( 15 – 17 ). Similarly, Syrian hamsters were treated with DMN followed by bile duct ligation also develop CCA ( 18 ). In these models, CCA develops ∼24 weeks after the experiment, with a yield rate of only 10%. One of the better characterized rat models of CCA has been the furan model described by Sirica et al ., which leads to the development of intestinal-type CCA in the caudate lobe of the liver ( 13 , 19 ).
TAA, which was originally used to preserve oranges, is a potent hepatotoxin and carcinogen ( 10 , 20 – 24 ). Prolonged administration of TAA causes hyperplastic liver nodules, liver cell adenomas and hepatocarcinomas. Various TAA administration methods have been used in experimental animals to produce liver cirrhosis and tumors, including intraperitoneal or subcutaneous administration ( 22 , 25 ), and administering the toxin with food ( 21 , 26 ) or drinking water ( 27 ). TAA is known as a potent hepatotoxicant that requires metabolic activation by mixed-function oxidases. Cytochrome P450 2B, 2E1 and flavin monooxygenase metabolize TAA to its toxic metabolites ( 28 ). TAA also induces toxic effects in the pancreas ( 29 ) and kidney ( 24 ). Although the molecular mechanisms that cause these toxic effects in different organs are not understood, TAA is known to interfere with ribosomal activity, thus hindering protein synthesis ( 29 ). More recently, acute TAA administration has been shown to stimulate DNA synthesis ( 30 ).
Although TAA-induced hepatic pathology is well characterized, only a few reports have focused on the role of orally administered TAA in the induction of biliary disease (dysplasia and/or CCA). Praet et al . reported a universal incidence of CCAs after prolonged (12 months) administration of TAA in male albino rats ( 30 ); the CCAs developed on a backdrop of liver cirrhosis and a variety of benign tumors such as angiomas and cystadenomas. Al-Bader et al . ( 14 ) reported that oral administration of 0.05% TAA could induce liver cirrhosis in Wistar rats at 12 weeks; by the 13th to 14th weeks, ‘severe’ bile duct proliferation and eventually invasive CCA with stromal desmoplasia could be induced. This report, however, did not characterize the histopathology of the biliary disease, or the frequency of CCA. The discrepancy in the onset of CCAs between the two reports ( 14 , 31 ) most probably reflects differences in the genetic backgrounds of the animals used and dose-dependent toxicities on the hepato-biliary system. Our study is one of the first to systematically document the time course and histopathology of TAA-induced biliary disease in rats. We demonstrate that oral administration of TAA in drinking water to male SD rats results in a multi-step model of biliary dysplasia and invasive CCA, which closely mimics human disease. Similar to preneoplastic lesions described in human CCAs ( 3 – 6 ), the rat cholangiolar epithelium displays a phase of progressive ‘biliary dysplasia’ preceding invasive cancer. In addition, both the precancerous and neoplastic biliary epithelia demonstrate foci of intestinal metaplasia (goblet cells), another well-known feature of the human counterpart ( 3 ). The strong, diffuse expression of biliary cytokeratin (CK19) confirms the bile ductular ontogeny of the neoplastic cells. The course of events is fairly reproducible in our carcinogenesis model, with a 50% yield rate of invasive CCA by the 16th week; by the 22nd week, the yield of invasive CCA is 100%. Notably, the occurrence of biliary dysplasia and invasive CCA precedes the development of hepatic fibrosis by 4 weeks, arguing against a ‘secondary’ biliary proliferation in response to cirrhosis.
The molecular alterations involved in human CCA tumorigenesis have not been well investigated. However, similar to a previously described furan model of rat cholangiocarcinogenesis ( 13 , 19 , 32 ), the receptor tyrosine kinases c-Met and c-erb-B2 were over-expressed in the neoplastic glands of invasive CCAs, while expression was absent or minimal in normal cholangiolar epithelium. Notably, striking up-regulation of both oncogenes was noted in the epithelial cells of dysplastic glands as early as in the 9th week of therapy, and persisted into the invasive phase of CCA. Stimulation of c-met via its ligand hepatocyte growth factor, also known as scatter factor (HGF/SF), leads to a plethora of biological and biochemical effects in the cell ( 33 ). There is increasing evidence to suggest that alterations in the kinases c-erb-B2 and c-met, together with possible aberrant autocrine expression of HGF/SGF, may be playing important roles associated with the development and/or progression of human CCA ( 7 – 9 , 34 ). Hence, evidence of up-regulation of these critical proto-oncogenes in our rat model, especially in the precancerous stages, provides strong reiteration of its comparability with human disease, as well as an avenue for interventional studies.
In conclusion, this study demonstrates that male SD rats fed with 300 mg/l TAA in drinking water provides an easy and reproducible animal model recapitulating the multi-stage progression of human CCA. The TAA rat model may serve as a powerful pre-clinical platform for both therapeutic strategies in invasive CCA, as well as for evaluating rational chemoprevention strategies in the dysplastic biliary epithelium.
To whom correspondence should be addressed Email: ycn@adm.cgmh.org.tw
This work was supported by Chang Gung Medical Research Program (CMRP) grant 10941289 to Dr Chun-Nan Yeh. A.M. is supported by the Johns Hopkins Clinical Scientist Award and the family of Margaret Lee.
References
Ahrendt,S.A., Nakeeb,A. and Pitt,H.A. (
de Groen,P.C., Gores,G.J., LaRusso,N.F., Gunderson,L.L. and Nagorney, D.M. (
Albores-Saavedra,J., Henson,D.E. and Sobin,L.H. (
Bergquist,A., Glaumann,H., Stal,P., Wang,G.S. and Broome,U. (
Fleming,K.A., Boberg,K.M., Glaumann,H., Berquist,A., Smith,D. and Clausen,O.P. (
Shimonishi,T., Sasaki,M. and Nakanuma,Y. (
Terada,T., Nakanuma,Y. and Sirica,A.E. (
Endo,K., Yoon,B.I., Pairojkul,C., Demetris,A.J. and Sirica,A.E. (
Hansel,D.E., Rahman,A., Hidalgo,M. et al . (
Dasgupta,A., Chatterjee,R. and Chowdhury,J.R. (
Park,T.J., Kim,H.S., Byun,K.H., Jang,J.J., Lee,Y.S. and Lim,I.K. (
Tsujimoto,T., Kuriyama,S., Tominaga,K. et al . (
Elmore,L.W. and Sirica,A.E. (
Al-Bader,A., Mathew,T.C., Abul,H., Al-Sayer,H., Singal,P.K. and Dashti,H.M. (
Lee,J.H., Rim,H.J. and Sell,S. (
Thamavit,W., Bhamarapravati,N., Sahaphong,S., Vajrasthira,S. and Angsubhakorn,S. (
Flavell,D.J. and Lucas,S.B. (
Cheifetz,R.E., Davis,N.L. and Owen,D.A. (
Sirica,A.E. (
Fitzhugh,O.G. and Nielson,A.A. (
Gupta,D.N. (
Dashti,H., Jeppsson,B., Hagerstrand,I., Hultberg,B., Srinivas,U., Abdulla,M., Joelsson,B. and Bengmark,S. (
Kuriyama,S., Tsujimoto,T., Nakatani,Y. et al . (
Al-Bader,A.A., Mathew,T.C., Al-Mosawi,M., Dashti,H.M., Kumar,D. and Singal,P.K. (
Gallagher,C.H., Gupta,D.N., Judah,J.D. and Rees,K.K. (
Muller,D., Zimmerman,S.I. and Schiller,F. (
Dashti,H.M., Mathew,T.C., Jadaon,M.M. and Ashkanani,E. (
Lee,J.W., Shin,K.D., Lee.,M., Kim,E.J., Han,S.S., Han,M.Y., Ha,H., Jeong,T.C. and Koh,W.S. (
Barker,E.A. and Smuckler,E.A. (
Morley,C.G. and Boyer,J.L. (
Praet,M.M. and Roels,H.J. (
Radaeva,S., Ferreira-Gonzalez,A. and Sirica,A.E. (
Maulik,G., Shrikhande,A., Kijima,T., Ma,P.C., Morrison,P.T. and Salgia,R. (
Author notes
1Department of Surgery and 2Department of Pathology, Chang Gung Memorial Hospital, Chang Gung University, Taiwan and 3Department of Pathology, Johns Hopkins University, Baltimore, MD, USA