-
PDF
- Split View
-
Views
-
Cite
Cite
Junichi Inokuchi, Alice Lau, Darren R. Tyson, David K. Ornstein, Loss of annexin A1 disrupts normal prostate glandular structure by inducing autocrine IL-6 signaling, Carcinogenesis, Volume 30, Issue 7, July 2009, Pages 1082–1088, https://doi.org/10.1093/carcin/bgp078
Close - Share Icon Share
Abstract
Annexin A1 (ANXA1) expression is commonly reduced in premalignant lesions and prostate cancer, but a causal relationship of ANAX1 loss with carcinogenesis has not been established. ANXA1 levels have been shown to inversely correlate with interleukin 6 (IL-6) expression in other cell types and IL-6 has been suggested to enhance prostate cancer initiation and promotion. To investigate whether loss of ANXA1 may contribute to prostate carcinogenesis, ANXA1 expression was reduced using RNA interference in non-tumorigenic human prostatic epithelial cells (RWPE-1/rA1). No effect on morphology, apoptosis, migration or anchorage-dependent or -independent growth was detected. However, IL-6 mRNA and secreted protein levels were elevated in RWPE-1/rA1 cells. In addition, re-expression of ANXA1 in these cells suppressed IL-6 secretion, and altering ANXA1 levels in prostate cancer cells had similar effects on IL-6. The effects of ANXA1 loss and increased IL-6 expression on prostate epithelium were examined using an assay of acinar morphogenesis in vitro . Acini formed by RWPE-1/rA1 cells had delayed luminal clearing and larger mean diameters than control cells. The RWPE-1/rA1 phenotype was recapitulated by treating control cells with recombinant IL-6 and was reversed in RWPE-1/rA1 cells by blocking IL-6 bioactivity. Taken together, these data support a direct role for decreased ANXA1 expression in prostate carcinogenesis and enhancing tumor aggressiveness via the upregulation of IL-6 expression and activity.
Introduction
Annexin A1 (ANXA1) is a member of a family of calcium-dependent phospholipid-binding proteins that has several known functions involving cellular membrane trafficking ( 1 ). Altered expression of ANXA1 is commonly found in numerous types of cancer ( 2 ), and loss or reduction of ANXA1 expression is a very common finding in prostatic intraepithelial neoplasia and invasive prostate cancer. Decreased ANXA1 protein expression in prostate cancer was first demonstrated by molecular profiling studies of human prostate cancer samples ( 3 ), and subsequent confirmatory studies demonstrated that ANXA1 protein expression is decreased in >90% of prostatic intraepithelial lesions and early stage prostate cancer ( 4 ). Expression of ANXA1 protein is further decreased in androgen-independent prostate cancer ( 5 , 6 ). Gene expression studies utilizing cDNA microarrays have yielded results consistent with immunohistochemical studies, suggesting that ANXA1 dysregulation occurs at the transcriptional level ( 7 ). Collectively, these studies suggest that decreased ANXA1 expression may affect prostate cancer initiation and progression, although a causal role has yet to be established.
Interleukin 6 (IL-6) is a pleiotropic cytokine initially characterized as a mediator of inflammation and other immune responses ( 8 ). Recent studies have implicated IL-6 and its major effector signal transducer and activator of transcription 3 (STAT3) as tumorigenic agents in many cancers, including breast, lung, colon, ovary and prostate cancer ( 9 ), and constitutively active STAT3 has been shown to be sufficient to induce prostate tumorigenesis ( 10 ), although the activation of STAT3 has also been shown to have prostate tumor-suppressive activity ( 11 ). Several investigators have demonstrated the involvement of IL-6 in proliferation, metastasis and androgen independence of prostate cancer cells as well as the prognosis and survival of patients ( 12 , 13 ). Relevance of IL-6 expression to carcinogenesis has been supported by recent studies investigating the role of IL-6 in lung ( 14 ) and breast ( 15 , 16 ) adenocarcinomas.
IL-6 has also been suggested as an important regulator of development and progression of prostate cancer ( 17 ). In the prostate, the expression levels of IL-6 and its receptor, a heterodimeric IL-6R–gp130 complex, increase during prostate carcinogenesis and progression ( 18 ), and the concomitant increase in receptor and ligand has been shown to create a functional autocrine loop ( 19 ). In vitro studies have shown that IL-6 is strongly expressed by androgen-independent prostate cancer cell lines such as DU145 and PC-3 cells and directly contributes to the proliferation of these cells in an autocrine manner ( 20 ). In addition, serum IL-6 levels are increased in patients with metastatic cancer ( 21 ) and correlate with tumor burden as assessed by serum prostate specific antigen or clinically evident metastases ( 22 ). Several investigators have suggested the involvement of IL-6 in proliferation, metastasis, androgen independence and the prognosis and survival of human patients with prostate cancer ( 12 , 13 ), and its association with morbidity has made IL-6 a candidate for targeted therapy of metastatic prostate cancer, even though the mechanisms leading to IL-6 expression by prostate cancer cells are not well understood. Although there is significant evidence for a role for IL-6 enhancing tumor aggressiveness, the role of increased IL-6 expression in the earliest events of prostate carcinogenesis has not been extensively studied and the mechanisms leading to the increased IL-6 expression in prostate cancer have not been determined.
Recent studies using Anxa1 -deficient mice have demonstrated that IL-6 expression is increased in the absence of ANXA1 ( 23–25 ). However, the regulatory role of ANXA1 on IL-6 expression in epithelial cells is not clear and that role in human cells is currently unknown. Thus, we hypothesized that ANXA1 deficiency in prostatic epithelial cells would lead to enhanced IL-6 expression and may contribute to tumorigenesis. Here, we demonstrate that, similar to results in the ANXA1-deficient mice, the reduction of ANXA1 expression induces IL-6 expression in benign and malignant human prostatic epithelial cells. Importantly, the enhanced secretion of IL-6 affects prostatic acinar morphogenesis concomitant with activation of STAT3. These studies provide evidence that decreased ANXA1 expression is an important mediator of IL-6 expression in prostate cancer and suggest that decreased ANXA1 expression may contribute to enhanced IL-6 secretion in other tumor types.
Materials and methods
Reagents and antibodies
Recombinant human epidermal growth factor (EGF) and tumor necrosis factor-α (TNF-α), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, 4′,6-diamidino-2-phenylindole and anti-β-actin antibodies were purchased from Sigma–Aldrich (St Louis, MO). Recombinant human IL-6 was from PeproTech (Rocky Hill, NJ). Anti-human IL-6 for neutralization of IL-6 bioactivity was purchased from R&D Systems (Minneapolis, MN). The ANXA1-specific antibody was purchased from Zymed Laboratories (San Francisco, CA). Anti-ANXA2 was from BD Biosciences (San Jose, CA). All other antibodies used for western blotting were from Cell Signaling Technologies (Danvers, MA).
Cell culture
Unless otherwise noted, all cell culture reagents and original plasmids were from Invitrogen (Carlsbad, CA). RWPE-1, RWPE-2 and DU145 cells were obtained from the American Type Culture Collection (Manassas, VA). RWPE-1 cells were cultured in keratinocyte serum-free medium containing 5 ng/ml EGF and 25 mg/ml bovine pituitary extract and 1% penicillin/streptomycin solution (P/S) (K-SFM). For EGF or TNF-α stimulation, cells were cultured in K-SFM medium containing 1% P/S without EGF and bovine pituitary extract (K-SFM basal) overnight and treated with ligand for the indicated times. DU145 cells were cultured in RPMI medium 1640 (Mediatech, Herndon, VA) containing 10% fetal bovine serum and 1% P/S. For serum starvation and generation of conditioned medium, RPMI medium 1640 containing 0.1% bovine serum albumin and 1% P/S was used. Western blotting, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assays for proliferation and soft agar colony formation assays (for anchorage-independent growth) have been described previously ( 26 ). Three-dimensional culture conditions have also been described ( 27 ). Secreted IL-6 was measured by standard enzyme-linked immunosorbent assay (Biolegend, San Diego, CA).
Reduction of ANXA1 expression
A lentiviral vector for the expression of short hairpin RNA (shRNA) was generated by subcloning the human U6 promoter, tandem BsmBI restriction digestion sites and U6 termination sequence into pLenti4/V5-DEST or pLenti6/V5-DEST (both from Invitrogen Corp., Carlsbad, CA) in the place of the CMV promoter region through the att B2 sequence (pLenti4/U6-term or pLenti6/U6-term). To generate an shRNA-expressing construct targeting ANXA1, double strand DNA was made by annealing complementary oligonucleotides and ligating into pLenti4/U6-term or pLenti6/U6-term at the BsmBI sites. Several shRNA sequences were tested for ANXA1 reduction by reverse transcription–polymerase chain reaction (PCR) and western blotting, and the most effective single shRNA was used for further experiments. For non-silencing control (NC), an shRNA sequence targeting ANXA2 that was not effective at reducing either ANXA2 or ANXA1 at the mRNA or protein level was used. The ANXA1-specific shRNA was directed at GGACAGACGTAAACGTGTTCA. The control shRNA sequence used was GCATAGCAACTTCGGATTTCA. Recombinant replication-incompetent lentiviruses were prepared according to the manufacturer's protocol (ViraPower, Invitrogen Corp.). Cells were selected by blasticidin at 4 μg/ml for 14 days. DU145-derived ANXA1-reduced cells were generated using pLenti4-based constructs and selected by zeocin at 40 μg/ml for 14 days. Several colonies were picked using pipette tips under the microscope for ANXA1-reduced cells (DU145/rA1.5 and rA1.7). Control cells were collected as a pool (DU145/NC.p).
ANXA1 expression constructs
A full-length human ANXA1 cDNA was first generated by PCR and cloned into pENTR/D-TOPO to generate pENTR-ANXA1. This plasmid was then recombined with pLenti6/V5-DEST to generate pLenti6-ANXA1. An shRNA-insensitive ANXA1 expression construct was generated by PCR-based site-directed mutagenesis to create GGACAGACGTAAA T GT C TTCAATACCATC. The resultant plasmid (pENTR-srANXA1) was then recombined with pLenti6/V5-DEST to generate pLenti6-srANXA1. All constructs were verified by DNA sequencing.
Isolation of RNA and reverse transcription–PCR
Total RNAs were isolated by using the RNeasy Mini kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized from 1 μg of total RNA by Omniscript Reverse Transcriptase (Qiagen), and PCR was performed using Taq DNA polymerase (Invitrogen) and gene-specific primers: for IL-6, 5′-AGCCACTCAC CTCTTCAGAA CGAATTG-3’ and 5′-CGTCAGCAGG CTGGCATTTG T-3’; for glyceraldehyde 3-phosphate dehydrogenase, 5′-ACCACAGTCC ATGCCATCAC-3’ and 5′-TCCACCACCCT GTTGCTGTA-3′. The optimal cycle number for each gene was determined empirically under non-saturating conditions.
Imaging
Phase-contrast images were performed using a Leica DM IRB inverted microscope with an attached Optronics 541510HC camera controlled by MicroFire software (Optronics, Goleta, CA). For confocal images, cells cultured in 3D were fixed using 2.0% formaldehyde in phosphate-buffered saline. Nuclei were stained using 0.5 ng/ml of 4′,6-diamidino-2-phenylindole for 5 min at room temperature and the wells were washed several times with phosphate-buffered saline at room temperature. Imaging was performed using a Zeiss LSM510 Laser Scanning Confocal Microscope and LSM Image Examiner software (Carl Zeiss MicroImaging, Thornwood, NY).
Quantitation of acinar structures
An acinar structure is defined here as a spheroid shaped cluster of cells with no apparent protrusions from the exterior surface. Other multicellular structures were excluded from the analysis. Classification of acinar structures with or without hollow lumen was determined using phase-contrast microscope images. The diameter of structure was scaled using LSM Image Examiner software (Carl Zeiss MicroImaging). At least 50 acinar structures were counted for each analysis.
Statistical analysis
All statistical analyses were two sided and were performed using GraphPad InStat version 3.0b for Macintosh (GraphPad Software, San Diego, CA; http://www.graphpad.com ). Values are expressed as the mean ± SD of three independent experiments and significant differences were determined using Student's t -test. Each experiment was done at least three times.
Results
Establishment of ANXA1-reduced derivative of RWPE-1 cells
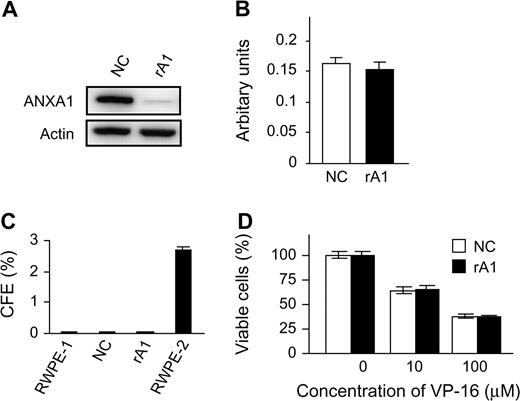
To investigate the possible contribution of decreased ANXA1 expression to prostate carcinogenesis, ANXA1-reduced cells were established by stable expression of shRNA in RWPE-1, an immortalized but non-tumorigenic human prostatic epithelial cell line (RWPE-1/rA1; Figure 1A ). ANXA1 has previously been demonstrated to affect cellular proliferation, differentiation and apoptosis in various cell types, and we previously reported a proapoptotic effect of ANXA1 in androgen-sensitive prostate cancer cell lines ( 26 ). Thus, each of these cellular activities was analyzed using traditional in vitro assays. Reduction of ANXA1 expression did not affect cellular morphology (data not shown) or proliferation rate under normal ( Figure 1B ) or growth factor-reduced conditions (data not shown). Likewise, loss of ANXA1 expression did not affect anchorage-independent growth as determined by colony formation in soft agar ( Figure 1C ). To determine whether decreased ANXA1 expression affects apoptosis, several apoptotic stimuli, including growth factor starvation, detachment from the substratum, hydrogen peroxide, calcium ionophore, TNF-α and etoposide, were tested for their effects on RWPE-1/rA1 cells. Surprisingly, the apoptotic response to each of these stimuli was not significantly different between the RWPE-1/rA1 and either parental RWPE-1 cells or the RWPE-1/NC cells ( Figure 1D ; data not shown).
Establishment of ANXA1-reduced cells and assessment of malignant characteristics. ( A ) Immunoblot of RWPE-1/NC and RWPE-1/rA1 cells. Equal protein loading was verified using antibodies to β-actin (Actin). ( B ) Proliferation rates were determined by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide dye conversion after equal numbers of cells were cultured for 72 h in normal growth medium. ( C ) Anchorage-independent growth was determined by counting colonies >200 μm in diameter that resulted after culture in soft agar for 21 days. Colony-forming efficiency (CFE) of each cell line analyzed is shown. RWPE-2 is a Ras-transformed variant of RWPE-1 ( 28 ) and used as a positive control. ( D ) VP-16 (etoposide)-induced apoptosis was measured using trypan blue exclusion to determine cellular viability after exposure to the indicated concentrations of VP-16 for 48 h. Data are presented as the mean ± SD of three independent experiments.
Reducing ANXA1 expression alters morphology of structures in 3D culture
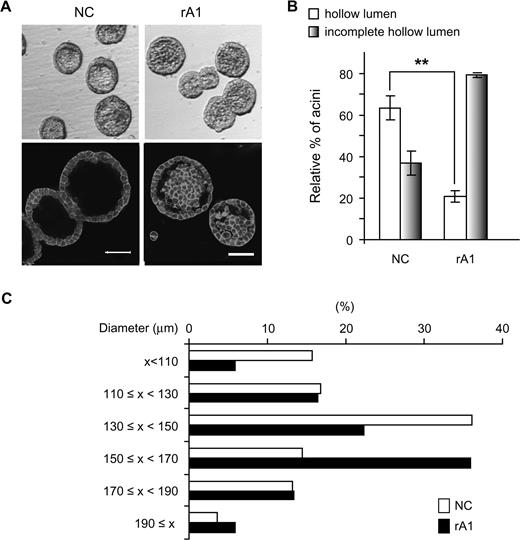
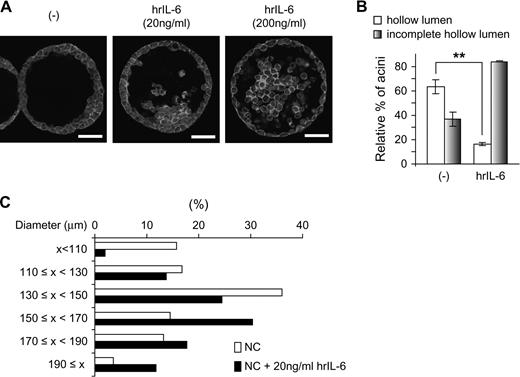
Recently, we reported on the optimized medium composition that allows RWPE-1 cells to undergo acinar morphogenesis in 3D culture in Matrigel and form consistent structures representative of normal adult human prostate glands ( 27 ). In this model, 4–5 days after seeding RWPE-1 cells in matrigel, two distinct populations of cells, an outer layer of cells in direct contact with the matrix and an inner subset of cells lacking matrix context, become evident within each spheroid (D.R.Tyson, J.Inokuchi and D.K.Ornstein, in preparation). The centrally located cells undergo caspase-mediated apoptosis beginning around day 6–7 ( 27 ), which contributes to the formation of complete hollow lumens by day 8–10. As expected, RWPE-1 cells with stable integration of an empty vector (RWPE-1/NC) behave similarly to RWPE-1 in this model, with >60% of acinar structures demonstrating complete hollow lumens by day 9 ( Figure 2B ). In contrast to the well-formed lumens of the control RWPE-1 cells, the structures formed by the rA1 cells had numerous cells remaining within their lumens that persisted beyond 7–10 days, and only ∼20% of acinar structures from rA1 cells had completely hollow lumens on day 9, indicating that reducing ANXA1 causes delays of luminal clearing. In addition, the sizes of acinar structures formed by the rA1 cells were larger than those of NC cells, with average diameters of 140.8 ± 31.0 μm (mean ± SD) versus 149.8 ± 24.3 μm ( P = 0.041), respectively ( Figure 2C ).
Delay of hollow lumen formation in 3D culture. ( A ) Acini formed by RWPE-1/rA1 cells showed delayed luminal clearing compared with parental RWPE-1 or RWPE-1/NC cells. Micrographs were generated of acinar structures visualized by phase-contrast microscopy (top) or of structures that were fixed, stained with 4′,6-diamidino-2-phenylindole to stain nuclein acids and rhodamine-phalloidin to label filamentous actin, and visualized by confocal fluorescence microscopy (bottom). Bar, 50 μm. ( B ) Acini with complete or incomplete hollow lumens from NC or rA1 cells were enumerated and presented as a mean percentage ± SD of total counted, ** P < 0.01. ( C ) The diameters of acini were measured and represented as percentage of total counted; at least 100 acini were counted for each cell line. The color version of this figure is available at Carcinogenesis Online.
IL-6 secretion is increased in response to ANXA1 reduction
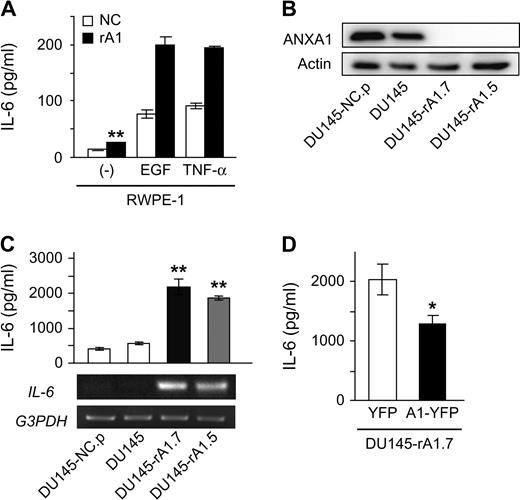
Recent studies have demonstrated that IL-6 expression is increased in the absence of ANXA1 in some cell types ( 23–25 ). Similarly, protein and mRNA levels of IL-6 were increased in rA1 cells and these levels were significantly enhanced in the presence of EGF or TNF-α ( Figure 3A ), known inducers of IL-6 expression ( 9 , 14 ). Secretion of IL-6 in unstimulated cells was increased 1.8-fold in rA1 cells relative to NC cells ( P < 0.01) and induction of IL-6 secretion under either EGF or TNF-α stimulation was enhanced 2.5-fold and 2.1-fold in rA1 cells relative to NC cells, respectively ( Figure 3A ).
ANXA1 regulates IL-6 expression in prostatic epithelial cells. ( A , C and D ) Secreted IL-6 levels were measured from culture supernatants of RWPE-1/NC and RWPE-1/rA1 cells by enzyme-linked immunosorbent assay and data represent the mean ± SD. (graphs). ( A ) IL-6 secretion was measured from unstimulated RWPE-1/NC and rA1 cells (−) or cells stimulated with 25 ng/ml EGF or 10 ng/ml TNF-α. Unstimulated IL-6 secretion from RWPE-1/rA1 was significantly different from unstimulated RWPE-1/NC by a Student's t -test, ** P = 0.0016. ( B ) Reduction of ANXA1 protein expression in DU145-expressing ANXA1 shRNA and controls was measured by western blotting. ( C ) IL-6 secretion from DU145 variants was determined by enzyme-linked immunosorbent assay (top) and IL-6 mRNA was estimated by semiquantitative reverse transcription (RT)–PCR (bottom). Double asterisks indicate significant difference from control DU145 cells as determined by Tukey–Kramer multiple comparison test ( P < 0.001). ( D ) Restoration of ANXA1 protein expression suppresses IL-6 secretion. DU145-rA1.7 cells were transiently transfected with yellow fluorescent protein (YFP) or shRNA-insensitive ANXA1-YFP (srA1) expression plasmids and secreted IL-6 levels were measured after 24 h in culture.
To determine whether the induction of IL-6 expression is a general response to ANXA1 loss and whether decreased ANXA1 expression alters IL-6 secretion in prostate cancer cells, ANXA1 expression was decreased in DU145 prostate cancer cells, which already secrete high levels of IL-6 ( 29 ). Several clones were isolated in which ANXA1 expression was decreased (DU145/rA1) and pooled cells infected with control viruses (DU145/NC.p) were generated. ANXA1 protein expression was decreased >95% in these cells compared with controls ( Figure 3B ), and IL-6 mRNA and secreted protein levels were significantly higher in DU145/rA1 cells relative to DU145/NC.p cells ( P < 0.01; Figure 3C ). Re-expression of shRNA-insensitive ANXA1 in DU145-rA1.7 suppressed IL-6 secretion ( Figure 3D ), demonstrating that the increased IL-6 expression was not due to off target effects of the shRNA construct used in this study and that ANXA1 expression levels regulate IL-6 expression.
We have recently shown that decreased ANXA2 expression in DU145 cells reduced the level of IL-6 expression and decreased the anchorage-dependent and -independent growth rates of DU145 cells ( 30 ). To rule out a role of altered ANXA2 expression as a cause for IL-6 expression, ANXA2 levels were examined in both RWPE-1/rA1 and DU145/rA1. ANXA2 levels were not affected by the loss of ANXA1 in either cell type and are maintained at the level found in the parental cells (data not shown), suggesting against a role for ANXA2 expression in modulating IL-6 levels in these cells.
IL-6 secretion in response to decreased ANXA1 expression results in autocrine activation of STAT3
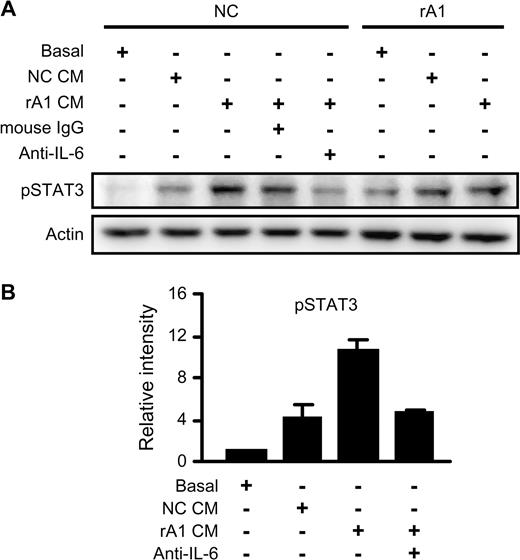
IL-6 is an autocrine growth factor for human prostate carcinoma cells ( 20 ), and IL-6 expression is enhanced by the activation of the IL-6 receptor ( 8 ). Thus, we sought to determine whether the increased secretion of IL-6 in rA1 cells was acting in an autocrine manner on the RWPE-1/rA1 cells. To test this, we examined the activation of STAT3, a major downstream target of IL-6 receptor signaling pathway. Conditioned medium from RWPE-1/rA1 cells significantly induced phosphorylation of STAT3 at Y705 in NC cells compared with basal medium or conditioned medium from NC cells ( Figure 4 ). Furthermore, this activation was suppressed by addition of an IL-6 function-neutralizing antibody but not by mouse IgG ( Figure 4 ). These results indicate that reducing ANXA1 expression in prostatic epithelial cells induces autocrine activity of IL-6 resulting in the activation of STAT3.
Autocrine IL-6 secretion induces phosphorylation of STAT3. ( A ) Conditioned medium (CM) was collected from RWPE-1/NC and rA1 cells cultured in basal medium for 24 h (NC CM and rA1 CM, respectively). Fresh cells (NC or rA1 as indicated on top) were washed with phosphate-buffered saline and incubated for 6 h in fresh K-SFM basal medium or conditioned medium from NC or rA1 cells. Mouse IgG as control or mouse anti-IL-6 was added at 1 μg/ml to block IL-6 activity in the indicated samples. Medium and additional IgG added to each sample are listed on left and indicated by ‘+’. Lysates were prepared and analyzed for phosphorylation of STAT3 (Y705) (pSTAT3) by immunoblotting. A representative experiment of three independent experiments is shown. ( B ) Relative band intensities of pSTAT3 in RWPE-1/NC cells were determined in relation to cells treated with basal medium for each of the three experiments. Bars represent the mean ± SD.
Autocrine activity of IL-6 affects hollow lumen formation
Although IL-6 is considered an autocrine growth factor for prostate cancer cell lines, the enhanced IL-6 expression in rA1 cells does not appear to affect anchorage-dependent and -independent proliferation rates as evidenced by results in Figure 1 . However, exogenous IL-6 administration (20 ng/ml) enhances proliferation similarly in rA1 and NC cells (data not shown). Thus, IL-6 secreted into the medium by rA1 cells may not be reaching a sufficiently high concentration to induce an effect using traditional in vitro assays of proliferation. To further investigate the possible role of ANXA1-induced IL-6 expression on prostate cells, we examined IL-6 levels in cells cultured in 3D. Similar to that seen when grown in 2D culture conditions, IL-6 secretion was enhanced in RWPE-1/rA1 cells when they were cultured in 3D to form acinar structures (data not shown). To determine if increased IL-6 levels may be causing the delayed luminal clearing and increased acinar diameters in the RWPE-1/rA1 cells, we first attempted to recapitulate the phenotype in control cells by adding recombinant IL-6 into the medium of control RWPE-1 cells (RWPE-1/NC) cultured in 3D. The addition of IL-6 delayed luminal clearing in a dose-dependent manner ( Figure 5A and B ). In addition, the sizes of acinar structures formed by IL-6-treated RWPE-1/NC cells were larger than those of untreated cells ( Figure 5C ). Thus, exogenous administration of IL-6 induced a phenotype of control RWPE-1 cells in 3D culture highly similar to that observed for RWPE-1/rA1 cells (compare Figures 2 and 5 ).
Effects of exogenous IL-6 on morphology of control RWPE-1 cells in 3D culture. ( A ) RWPE-1/NC cells were cultured in 3D with or without the indicated amounts of human recombinant (hr) IL-6 for 9 days, fixed, stained with 4′,6-diamidino-2-phenylindole (blue) to detect nuclei and rhodamine–phalloidin (red) to detect filamentous actin. Cells were visualized using confocal fluorescence microscopy. Bar, 50 μm. ( B and C ) Hollow lumen formation and acinar diameters of RWPE-1/NC cells cultured in 3D in the absence or presence of 20 ng/ml IL-6 were determined as in Figure 2 . The data are presented as mean percentage ± SD of total structures counted. Student's t -test, ** P < 0.01. The color version of this figure is available at Carcinogenesis Online.
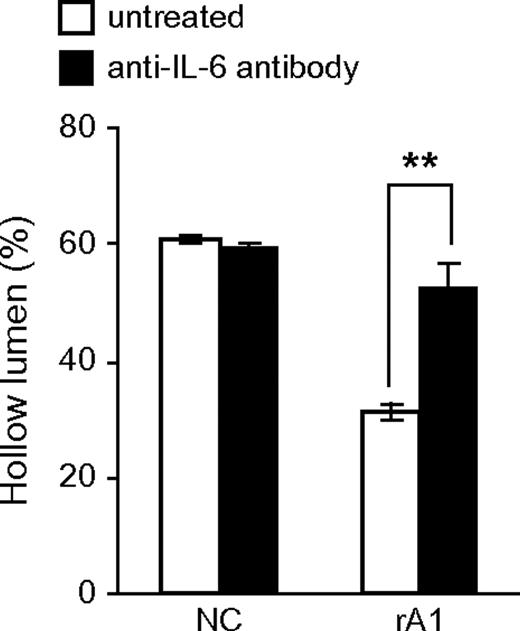
To directly test whether IL-6 secreted by RWPE-1/rA1 cells contributes to the altered phenotype in 3D culture, the cells were cultured in the presence of an IL-6-neutralizing antibody. The presence of the IL-6 antibody was sufficient to restore normal luminal clearing in the RWPE-1/rA1 cells ( Figure 6 ) and to reduce the mean acinar diameter (data not shown). These results were specific to IL-6 since control mouse IgG had minimal effects on these parameters (data not shown).
Blocking IL-6 bioactivity restores luminal clearing. RWPE-1/NC and rA1 cells were cultured in 3D in the presence or absence of 1 μg/ml anti-IL-6 antibody for 9 days and hollow lumen formation was quantified as before. Mean percentages ± SD of total structures counted are shown. Student's t -test, ** P < 0.01.
Discussion
Although the relevant biological function of ANXA1 is still not well understood, it has been proposed to be involved in diverse cellular functions, including regulation of membrane trafficking, signal transduction, exocytosis, inflammation, cell growth, differentiation and apoptosis ( 31 ). It appears likely that ANXA1 has some tumor-suppressive functions since absent or reduced levels of ANXA1 expression have been observed in several types of cancer, including B-cell lymphomas, squamous cell carcinomas of the esophagus and breast cancer, in addition to prostate cancer ( 2 ). Recently, we reported proapoptotic effects of ANXA1 in androgen-sensitive prostate cancer cell lines, LNCaP and MDA-PCa-2b ( 26 ), but a direct effect of decreased ANXA1 protein expression on carcinogenesis has yet to be established.
Carcinogenesis is a multistep process that requires dysregulated cell division, growth and survival, and one strategy used by cancer cells to affect these pathways is through autocrine production of growth and survival factors ( 15 ). IL-6 is a multifunctional cytokine that was originally characterized as a regulator of immune and inflammatory responses. However, increased IL-6 expression has been implicated in the modulation of growth and differentiation in many malignant tumors and has been associated with poor prognosis in several solid and hematopoietic neoplasms ( 8 , 32 ). Several studies have implicated IL-6 and its major effector STAT3 as protumorigenic agents in many cancers, including breast, lung, colon, ovarian, hematological and prostate cancer (reviewed in ref. 9 ). Recently two studies stressed the importance of autocrine IL-6 activity in lung and breast cancers and implicate IL-6 as an important activator of oncogenic STAT3 in lung adenocarcinoma ( 14 ) and of Jagged-1/Notch signaling in breast tumor mammospheres ( 33 ). Interestingly, in lung adenocarcinoma, mutant EGFR was demonstrated to be one cause of increased IL-6 expression ( 14 ). However, since EGFR mutations only occur in ∼10% of human cases, while IL-6/STAT3 activity is enhanced in ∼50% of cases, mechanisms other than activating EGFR mutations likely contribute to the enhanced IL-6 activity. Nevertheless, it is tempting to speculate that ANXA1 will play a role in regulating IL-6 levels in epithelial cells from these other organ sites as has been shown here for prostate.
A major finding of these studies is the fact that the effects of increased IL-6 expression were only detectable when cells were cultured in 3D. The reasons for this are not immediately clear but may involve several mechanisms. One possible mechanism involves the presence of extracellular matrix proteins surrounding the acini, from both exogenous and endogenous sources, which may act as a barrier to diffusion of IL-6. Alternatively, the microenvironment of the cells may alter their response to IL-6, i.e. IL-6 signaling is qualitatively different between the two cellular microenvironments. Preliminary studies have suggested that cells cultured in a 3D environment have markedly different response to EGF (D.R.Tyson, J.Inokuchi, A.Lau and D.K.Ornstein, in preparation), suggesting that IL-6 signal transduction may likewise be altered. Investigation of the signaling pathways induced by IL-6 in 3D-cultured RWPE-1 cells should help clarify the signaling pathways responsible for altered acinar morphogenesis and may provide a useful model system to investigate potentially tumorigenic IL-6-mediated signal transduction in vitro .
While the present studies have provided evidence for a tumorigenic effect of the loss of ANXA1 on prostate epithelial cells, there are a number of remaining questions to be addressed in future studies. First, the mechanisms by which IL-6 leads to delayed luminal clearing and increased acinar diameters are not clear. For example, it is possible that increased levels of IL-6 may provide resistance to apoptosis and/or increased proliferation within the context of organized prostatic epithelium; however, the specific roles of each of these with respect to the resultant acinar morphologies are unclear. The recapitulation of normal appearing and functioning acinar structures representative of normal mammary glands in 3D culture have provided a strong basis for comparative studies between 3D-cultured cells and mammary glands in vivo ( 34 ). The relevance of structures formed in 3D culture systems to clinical specimens has recently been confirmed by studies examining the transcription profiles of structures formed in 3D and comparing them to clinical breast cancer specimens ( 35 ). Remarkably, the expression profile of the growth arrested cells forming normal appearing structures in 3D culture was correlated with a better prognosis ( 35 ), strongly suggesting that the culture of cells in 3D truly represents salient features of the organ they are attempting to model. The in vitro models of mammary acinar morphogenesis have been used to examine several phenotypic characteristics of the resultant spheroid structures, such as lumen formation (luminal apoptosis/autophagy) ( 36–38 ), polarization ( 39–41 ) and differentiation ( 42 , 43 ), and altered regulation of each these activities has been associated with malignant phenotypes. However, it remains to be determined whether mammary epithelial cells and prostatic epithelial cells utilize similar or different mechanisms leading to the formation of the hollow spheroids. Preliminary results suggest that the mechanisms leading to lumen formation in prostatic epithelial cells are not the same as those of mammary epithelial cells (D.R.Tyson, unpublished data). Furthermore, the control of acinar diameter is not well understood in any model of spheroid formation in vitro .
Nevertheless, the increased expression and activity of IL-6 in response to ANXA1 loss directly contribute to delayed luminal clearing and increased acinar diameters. These effects are likely due to the activation of STAT3, which is a major regulator of cell proliferation and survival, particularly through its ability to regulate the expression of c-Myc, Mcl-1, cyclin D and Bcl-2 ( 15 , 44 ). Importantly, each of these factors has been implicated as an oncogene relevant to prostate cancer ( 45–48 ), providing a functional link between IL-6 and prostate carcinogenesis. The increased phosphorylation of STAT3 in the RWPE-1/rA1 cells may also be affected by alterations within the cell that augment the activation of this important transcriptional regulator in addition to the increased autocrine activity of IL-6. Several suppressors of cytokine signaling are expressed in RWPE-1 cells, including suppressors of cytokine signaling 3 ( 49 ), and IL-6-mediated signal transduction is likely affected by their relative expression levels. It is possible that suppressors of cytokine signaling 3 expression is reduced in response to loss of ANXA1 expression thereby leading to increased STAT3 phosphorylation. However, the ability of the function-blocking IL-6 antibody to reduce STAT3 phosphorylation to the levels found in parental RWPE-1 cells and to restore normal luminal clearing of the rA1 cells strongly suggest that autocrine IL-6 signaling is required for these effects.
IL-6 is known to activate other important signaling molecules besides STAT3, including extracellular-regulated kinases 1 & 2 and Akt ( 9 ), and these pathways may also contribute to the delay of luminal clearing and increased acinar size of RWPE-1 cells with reduced ANXA1 expression. However, extracellular-regulated kinases 1 & 2 activity in RWPE-1/rA1 cells was similar to parental RWPE-1 cells under basal- and EGF-treated conditions, suggesting against ANXA1 dysregulation affecting extracellular-regulated kinases 1 & 2 signaling in these cells (unpublished data). In addition, exogenously administered IL-6 does not induce Akt phosphorylation in RWPE-1 or RWPE-1/rA1 cells cultured in 2D on plastic dishes (unpublished data), suggesting against a role for Akt in the altered phenotype of the 3D-cultured cells.
Another area requiring further investigation involves the mechanisms by which decreased ANXA1 levels lead to enhanced expression and secretion of IL-6. One possible factor involved in this process relates to the well-known inhibitory function of ANXA1 on phospholipase A2 ( 50 ), a rate-limiting enzyme in the prostaglandin synthesis pathway ( 51 ). ANXA1 likely plays an important regulatory role during prostaglandin synthesis and its loss may lead to dysregulated expression and release of prostaglandins. Importantly, prostaglandin E2 has previously been shown to contribute to prostatic intraepithelial neoplasia through the IL-6/STAT3 signaling pathway ( 52 ), and functional interaction of ANXA1 with phospholipase A2 inhibits prostaglandin E2 synthesis in endothelial cells ( 53 ).
In summary, our data demonstrate that the reduction of ANXA1 expression induces IL-6 expression in benign and malignant human prostatic epithelial cells and support the possibility that alterations of ANXA1 expression may be an important regulator of IL-6 expression in a variety of human malignancies. The results presented here clearly demonstrate that IL-6 delays hollow lumen formation and increased acinar size of cells in 3D culture and that decreased expression of ANXA1 in prostatic epithelial cells induces similar effects through the autocrine activity of IL-6. Further investigation should elucidate the mechanisms by which ANXA1 levels affect IL-6 expression.
Supplementary material
The color version of Supplementary Data and Supplementary Data can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (DK068137 to D.K.O.); American Urological Association Foundation (to D.R.T.).
Abbreviations
- ANXA1
annexin A1
- EGF
epidermal growth factor
- IL-6
interleukin 6
- K-SFM
keratinocyte serum-free medium containing 5 ng/ml EGF and 25 mg/ml bovine pituitary extract and 1% penicillin/streptomycin solution (P/S)
- NC
non-silencing control
- PCR
polymerase chain reaction
- P/S
penicillin/streptomycin solution
- shRNA
short hairpin RNA
- STAT3
signal transducer and activator of transcription 3
- TNF-α
tumor necrosis factor-α
We would like to thank Vito Quaranta for his critical assessment of this manuscript. Author contributions: J.I., D.R.T. and D.K.O. designed experiments; J.I., A.L. and D.R.T. performed experiments; J.I., D.K.O. and D.R.T. analyzed data; J.I. and D.R.T. wrote the manuscript and D.K.O. edited the manuscript.
Conflict of Interest Statement: None declared.
References
Author notes
Sharing senior authorship.